Tumor necrosis factor-alpha acts as a complete mitogen for primary rat hepatocytes
- PMID: 12875968
- PMCID: PMC1868193
- DOI: 10.1016/s0002-9440(10)63676-0
Tumor necrosis factor-alpha acts as a complete mitogen for primary rat hepatocytes
Abstract
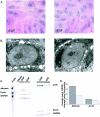
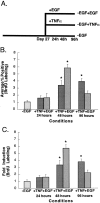
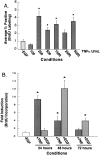
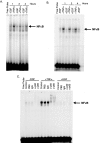
The cytokine tumor necrosis factor (TNF)-alpha has previously been shown to prime hepatocytes to a state of replicative competence, but has not been shown to act as a complete mitogen for these cells. In the present study we have altered our previously described long-term dimethyl sulfoxide culture system to exclude all known hepatocyte mitogens from the culture media and enable us to directly examine the effects of TNF-alpha on primary rat hepatocytes. We have shown that cells maintained under these culture conditions retain the biochemical and morphological features of well-differentiated hepatocytes. Treatment with TNF-alpha induced DNA synthesis relative to control, to a level not significantly different from that induced by the known hepatocyte mitogen, epidermal growth factor (EGF). Maximal DNA synthesis was induced by treatment with 250 U/ml TNF-alpha for 24 hours. Mitotic figures were observed in cultures treated with TNF-alpha or EGF but not in untreated controls. Treatment of cultures with TNF-alpha, but not EGF, induced activation of both nuclear factor-kappaB p50 homodimers and p50/p65 heterodimers. DNA synthesis induced by TNF-alpha was inhibited by treatment with transforming growth factor-beta. Based on the results of our studies, we conclude that TNF-alpha acts as a complete mitogen for rat hepatocytes.
Figures




Similar articles
-
Effects of mitogens and co-mitogens on the formation of small-cell colonies in primary cultures of rat hepatocytes.J Cell Physiol. 1993 Dec;157(3):461-8. doi: 10.1002/jcp.1041570305. J Cell Physiol. 1993. PMID: 8253857
-
Tumor necrosis factor (TNF) receptor-2-mediated DNA synthesis and proliferation in primary cultures of adult rat hepatocytes: The involvement of endogenous transforming growth factor-alpha.Eur J Pharmacol. 2009 Feb 14;604(1-3):12-9. doi: 10.1016/j.ejphar.2008.12.004. Epub 2008 Dec 9. Eur J Pharmacol. 2009. PMID: 19100731
-
Role of hepatic non-parenchymal cells in the response of rat hepatocytes to the peroxisome proliferator nafenopin in vitro.Carcinogenesis. 2000 Dec;21(12):2159-65. doi: 10.1093/carcin/21.12.2159. Carcinogenesis. 2000. PMID: 11133804
-
Genetic inactivation of RelA/p65 sensitizes adult mouse hepatocytes to TNF-induced apoptosis in vivo and in vitro.Gastroenterology. 2007 Jun;132(7):2489-503. doi: 10.1053/j.gastro.2007.03.033. Epub 2007 Mar 21. Gastroenterology. 2007. PMID: 17570221
-
Die another day.J Hepatol. 2003 Jun;38(6):873-5. doi: 10.1016/s0168-8278(03)00092-8. J Hepatol. 2003. PMID: 12763387 Review. No abstract available.
Cited by
-
Autocrine-controlled formation and function of tissue-like aggregates by primary hepatocytes in micropatterned hydrogel arrays.Tissue Eng Part A. 2011 Apr;17(7-8):1055-68. doi: 10.1089/ten.TEA.2010.0398. Epub 2011 Feb 2. Tissue Eng Part A. 2011. PMID: 21121876 Free PMC article.
-
An inducible autocrine cascade regulates rat hepatocyte proliferation and apoptosis responses to tumor necrosis factor-alpha.Hepatology. 2008 Jul;48(1):276-88. doi: 10.1002/hep.22335. Hepatology. 2008. PMID: 18536058 Free PMC article.
-
Regulation of the g1/s transition in hepatocytes: involvement of the cyclin-dependent kinase cdk1 in the DNA replication.Int J Hepatol. 2012;2012:689324. doi: 10.1155/2012/689324. Epub 2012 Oct 3. Int J Hepatol. 2012. PMID: 23091735 Free PMC article.
-
Tumor necrosis factor-induced toxic liver injury results from JNK2-dependent activation of caspase-8 and the mitochondrial death pathway.J Biol Chem. 2006 Jun 2;281(22):15258-67. doi: 10.1074/jbc.M512953200. Epub 2006 Mar 29. J Biol Chem. 2006. PMID: 16571730 Free PMC article.
-
The diverse roles of the TNF axis in cancer progression and metastasis.Trends Cancer Res. 2016 Jan 1;11(1):1-27. Trends Cancer Res. 2016. PMID: 27928197 Free PMC article.
References
-
- Loffreda S, Rai R, Yang SQ, Lin HZ, Diehl AM: Bile ducts and portal and central veins are major producers of tumor necrosis factor α in regenerating liver. Gastroenterology 1997, 112:2089-2098 - PubMed
-
- Akerman P, Cote P, Yang SQ, McClain C, Nelson S, Bagby GJ, Diehl AM: Antibodies to tumor necrosis factor-α inhibit liver regeneration after partial hepatectomy. Am J Physiol 1992, 263:G579-G585 - PubMed
-
- Webber EM, Bruix J, Pierce RH, Fausto N: Tumor necrosis factor primes hepatocytes for DNA replication in the rat. Hepatology 1998, 28:1226-1234 - PubMed
-
- Yamada Y, Webber EM, Kirillova I, Peschon JJ, Fausto N: Analysis of liver regeneration in mice lacking type I or type II TNF receptor: requirement for type I but not type II receptor. Hepatology 1998, 28:906-913 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

