Neuron-specific activation of murine cytomegalovirus early gene e1 promoter in transgenic mice
- PMID: 12875983
- PMCID: PMC1868196
- DOI: 10.1016/S0002-9440(10)63691-7
Neuron-specific activation of murine cytomegalovirus early gene e1 promoter in transgenic mice
Abstract
The brain is the main target in congenital cytomegalovirus (CMV) infection and immunocompromised patients. No definite evidence that a CMV has special affinity for the central nervous system (CNS) has been published. Here, we generated transgenic mice with an e1 promoter/enhancer region connected to the reporter gene lacZ. Surprisingly, expression of the transgene was completely restricted to the CNS in all lines of transgenic mice. The transgene was expressed in subpopulation of neurons in the cerebral cortex, hippocampus, diencephalon, brainstem, cerebellum, and spinal cord in all of the lines. Non-neuronal cells in the CNS were negative for transgene expression. Activation of the transgene was first observed in neurons of mesencephalon in late gestation, and then the number of positive neurons increased in various parts of the brain as development proceeded. Upon infection of the transgenic mouse brains with MCMV, the location of the activated neurons became more extensive, and the number of such neurons increased. These results suggest that there are host factor(s) that directly activate the MCMV early gene promoter in neurons. This neuron-specific activation may be associated with persistent infection in the brain and may be responsible for the neuronal dysfunction and neuronal cell loss caused by CMV infection.
Figures
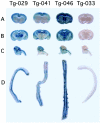
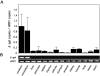




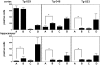
References
-
- Weller TH: The cytomegalovirus: ubiquitous agents with protean clinical manifestations. N Engl J Med 1971, 285:203-214 - PubMed
-
- Becroft DMO: Prenatal cytomegalovirus infection: epidemiology, pathology, and pathogenesis. Perspect Pediatr Pathol 1981, 6:203-241 - PubMed
-
- Stagno S, Pass RF, Cloud G, Bright WJ, Henderson RE, Walton PD, Veren DA, Page F, Alford CA: Primary cytomegalovirus infection in pregnancy: incidence, transmission to fetus, and clinical outocome. JAMA 1986, 256:1904-1908 - PubMed
-
- Ho M: Congenital and perinatal human cytomegalovirus infection. Ho M eds. Cytomegalovirus: Biology and Infection. 1991:pp 205-227 Plenum New York
-
- Pass RF, Stagno S, Myers GJ, Alford CA: Outcome of symptomatic congenital cytomegalovirus infection: results of long-term longitudinal follow-up. Pediatrics 1980, 66:758-762 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

