Escherichia coli single-stranded DNA-binding protein mediates template recycling during transcription by bacteriophage N4 virion RNA polymerase
- PMID: 12876194
- PMCID: PMC170904
- DOI: 10.1073/pnas.1133325100
Escherichia coli single-stranded DNA-binding protein mediates template recycling during transcription by bacteriophage N4 virion RNA polymerase
Abstract
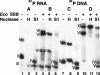
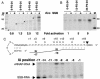
Coliphage N4 virion RNA polymerase (vRNAP), the most distantly related member of the T7-like family of RNA polymerases, is responsible for transcription of the early genes of the linear double-stranded DNA phage genome. Escherichia coli single-stranded DNA-binding protein (EcoSSB) is required for N4 early transcription in vivo, as well as for in vitro transcription on super-coiled DNA templates containing vRNAP promoters. In contrast to other DNA-dependent RNA polymerases, vRNAP initiates transcription on single-stranded, promoter-containing templates with in vivo specificity; however, the RNA product is not displaced, thus limiting template usage to one round. We show that EcoSSB activates vRNAP transcription at limiting single-stranded template concentrations through template recycling. EcoSSB binds to the template and to the nascent transcript and prevents the formation of a transcriptionally inert RNA:DNA hybrid. Using C-terminally truncated EcoSSB mutant proteins, human mitochondrial SSB (Hsmt SSB), phage P1 SSB, and F episome-encoded SSB, as well as a Hsmt-EcoSSB chimera, we have mapped a determinant of template recycling to the C-terminal amino acids of EcoSSB. T7 RNAP contains an amino-terminal domain responsible for binding the RNA product as it exits from the enzyme. No sequence similarity to this domain exists in vRNAP. Hereby, we propose a unique role for EcoSSB: It functionally substitutes in N4 vRNAP for the N-terminal domain of T7 RNAP responsible for RNA binding.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

