Profiling receptor tyrosine kinase activation by using Ab microarrays
- PMID: 12876202
- PMCID: PMC170918
- DOI: 10.1073/pnas.1633513100
Profiling receptor tyrosine kinase activation by using Ab microarrays
Abstract
Signal transduction in mammalian cells is mediated by complex networks of interacting proteins. Understanding these networks at a circuit level requires devices to measure the amounts and activities of multiple proteins in a rapid and accurate manner. Ab microarrays have previously been applied to the quantification of labeled recombinant proteins and proteins in serum. The development of methods to analyze intracellular signaling molecules on microarrays would make Ab arrays widely useful in systems biology. Here we describe the fabrication of multiplex Ab arrays sensitive to the amounts and modification states of signal transduction proteins in crude cell lysates and the integration of these arrays with 96-well microtiter plate technology to create microarrays in microplates. We apply the Ab arrays to monitoring the activation, uptake, and signaling of ErbB receptor tyrosine kinases in human tumor cell lines. Data obtained from multicolor ratiometric microarrays correlate well with data obtained by using traditional approaches, but the arrays are faster and simpler to use. The integration of microplate and microarray methods for crude cell lysates should make it possible to identify and analyze small molecule inhibitors of signal transduction processes with unprecedented speed and precision. We demonstrate the future potential of this approach by characterizing the action of the epidermal growth factor receptor inhibitor PD153035 on cells by using Ab arrays; direct scale-up to array-based screening in 96- and 384-well plates should allow small molecules to be identified with specific inhibitory profiles against a signaling network.
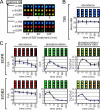
Figures



References
-
- DeRisi, J. L., Iyer, V. R. & Brown, P. O. (1997) Science 278, 680–686. - PubMed
-
- Wiese, R., Belosludtsev, Y., Powdrill, T., Thompson, P. & Hogan, M. (2001) Clin. Chem. 47, 1451–1457. - PubMed
-
- Ekins, R., Chu, F. & Biggart, E. (1990) Ann. Biol. Clin. (Paris) 48, 655–666. - PubMed
-
- Ekins, R. & Chu, F. (1992) Ann. Biol. Clin. (Paris) 50, 337–353. - PubMed
-
- Moody, M. D., Van Arsdell, S. W., Murphy, K. P., Orencole, S. F. & Burns, C. (2001) BioTechniques 31, 186–194. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

