Vacuole membrane fusion: V0 functions after trans-SNARE pairing and is coupled to the Ca2+-releasing channel
- PMID: 12876274
- PMCID: PMC2172786
- DOI: 10.1083/jcb.200212004
Vacuole membrane fusion: V0 functions after trans-SNARE pairing and is coupled to the Ca2+-releasing channel
Abstract
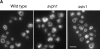
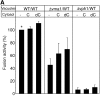
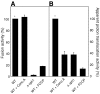
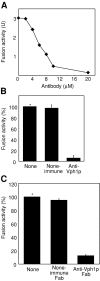
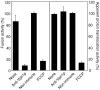
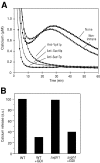
Pore models of membrane fusion postulate that cylinders of integral membrane proteins can initiate a fusion pore after conformational rearrangement of pore subunits. In the fusion of yeast vacuoles, V-ATPase V0 sectors, which contain a central cylinder of membrane integral proteolipid subunits, associate to form a transcomplex that might resemble an intermediate postulated in some pore models. We tested the role of V0 sectors in vacuole fusion. V0 functions in fusion and proton translocation could be experimentally separated via the differential effects of mutations and inhibitory antibodies. Inactivation of the V0 subunit Vph1p blocked fusion in the terminal reaction stage that is independent of a proton gradient. Deltavph1 mutants were capable of docking and trans-SNARE pairing and of subsequent release of lumenal Ca2+, but they did not fuse. The Ca2+-releasing channel appears to be tightly coupled to V0 because inactivation of Vph1p by antibodies blocked Ca2+ release. Vph1 deletion on only one fusion partner sufficed to severely reduce fusion activity. The functional requirement for Vph1p correlates to V0 transcomplex formation in that both occur after docking and Ca2+ release. These observations establish V0 as a crucial factor in vacuole fusion acting downstream of trans-SNARE pairing.
Figures







References
-
- Adachi, I., K. Puopolo, N. Marquez-Sterling, H. Arai, and M. Forgac. 1990. Dissociation, cross-linking, and glycosylation of the coated vesicle proton pump. J. Biol. Chem. 265:967–973. - PubMed
-
- Arai, H., S. Pink, and M. Forgac. 1989. Interaction of anions and ATP with the coated vesicle proton pump. Biochemistry. 28:3075–3082. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

