PAK1 phosphorylation of MEK1 regulates fibronectin-stimulated MAPK activation
- PMID: 12876277
- PMCID: PMC2172784
- DOI: 10.1083/jcb.200212141
PAK1 phosphorylation of MEK1 regulates fibronectin-stimulated MAPK activation
Abstract
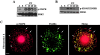
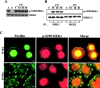
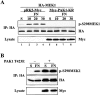
Activation of the Ras-MAPK signal transduction pathway is necessary for biological responses both to growth factors and ECM. Here, we provide evidence that phosphorylation of S298 of MAPK kinase 1 (MEK1) by p21-activated kinase (PAK) is a site of convergence for integrin and growth factor signaling. We find that adhesion to fibronectin induces PAK1-dependent phosphorylation of MEK1 on S298 and that this phosphorylation is necessary for efficient activation of MEK1 and subsequent MAPK activation. The rapid and efficient activation of MEK and phosphorylation on S298 induced by cell adhesion to fibronectin is influenced by FAK and Src signaling and is paralleled by localization of phospho-S298 MEK1 and phospho-MAPK staining in peripheral membrane-proximal adhesion structures. We propose that FAK/Src-dependent, PAK1-mediated phosphorylation of MEK1 on S298 is central to the organization and localization of active Raf-MEK1-MAPK signaling complexes, and that formation of such complexes contributes to the adhesion dependence of growth factor signaling to MAPK.
Figures
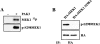





References
-
- Aplin, A.E., A.K. Howe, and R.L. Juliano. 1999. Cell adhesion molecules, signal transduction and cell growth. Curr. Opin. Cell Biol. 11:737–744. - PubMed
-
- Assoian, R.K., and M.A. Schwartz. 2001. Coordinate signaling by integrins and receptor tyrosine kinases in the regulation of G1 phase cell-cycle progression. Curr. Opin. Genet. Dev. 11:48–53. - PubMed
-
- Bagrodia, S., and R.A. Cerione. 1999. Pak to the future. Trends Cell Biol. 9:350–355. - PubMed
-
- Brugnera, E., L. Haney, C. Grimsley, M. Lu, S.F. Walk, A.C. Tosello-Trampont, I.G. Macara, H. Madhani, G.R. Fink, and K.S. Ravichandran. 2002. Unconventional Rac-GEF activity is mediated through the Dock180-ELMO complex. Nat. Cell Biol. 4:574–582. - PubMed
-
- Brunet, A., G. Pages, and J. Pouyssegur. 1994. Growth factor-stimulated MAP kinase induces rapid retrophosphorylation and inhibition of MAP kinase kinase (MEK1). FEBS Lett. 346:299–303. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

