The cytoplasmic domain of the Plasmodium falciparum ligand EBA-175 is essential for invasion but not protein trafficking
- PMID: 12876279
- PMCID: PMC2172798
- DOI: 10.1083/jcb.200301046
The cytoplasmic domain of the Plasmodium falciparum ligand EBA-175 is essential for invasion but not protein trafficking
Abstract
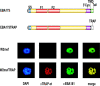
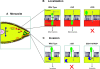
The invasion of host cells by the malaria parasite Plasmodium falciparum requires specific protein-protein interactions between parasite and host receptors and an intracellular translocation machinery to power the process. The transmembrane erythrocyte binding protein-175 (EBA-175) and thrombospondin-related anonymous protein (TRAP) play central roles in this process. EBA-175 binds to glycophorin A on human erythrocytes during the invasion process, linking the parasite to the surface of the host cell. In this report, we show that the cytoplasmic domain of EBA-175 encodes crucial information for its role in merozoite invasion, and that trafficking of this protein is independent of this domain. Further, we show that the cytoplasmic domain of TRAP, a protein that is not expressed in merozoites but is essential for invasion of liver cells by the sporozoite stage, can substitute for the cytoplasmic domain of EBA-175. These results show that the parasite uses the same components of its cellular machinery for invasion regardless of the host cell type and invasive form.
Figures



Similar articles
-
Erythrocyte-binding antigen 175 mediates invasion in Plasmodium falciparum utilizing sialic acid-dependent and -independent pathways.Proc Natl Acad Sci U S A. 2003 Apr 15;100(8):4796-801. doi: 10.1073/pnas.0730883100. Epub 2003 Apr 2. Proc Natl Acad Sci U S A. 2003. PMID: 12672957 Free PMC article.
-
Baculovirus-expressed Plasmodium reichenowi EBA-140 merozoite ligand is host specific.Parasitol Int. 2016 Dec;65(6 Pt A):708-714. doi: 10.1016/j.parint.2016.07.009. Epub 2016 Jul 19. Parasitol Int. 2016. PMID: 27443851
-
Plasmodium reichenowi EBA-140 merozoite ligand binds to glycophorin D on chimpanzee red blood cells, shedding new light on origins of Plasmodium falciparum.Parasit Vectors. 2017 Nov 7;10(1):554. doi: 10.1186/s13071-017-2507-8. Parasit Vectors. 2017. PMID: 29115972 Free PMC article.
-
How many pathways for invasion of the red blood cell by the malaria parasite?Trends Parasitol. 2003 Oct;19(10):430-2. doi: 10.1016/j.pt.2003.08.005. Trends Parasitol. 2003. PMID: 14519576 Review.
-
Erythrocyte glycophorins as receptors for Plasmodium merozoites.Parasit Vectors. 2019 Jun 24;12(1):317. doi: 10.1186/s13071-019-3575-8. Parasit Vectors. 2019. PMID: 31234897 Free PMC article. Review.
Cited by
-
A malaria parasite phospholipase facilitates efficient asexual blood stage egress.PLoS Pathog. 2023 Jun 23;19(6):e1011449. doi: 10.1371/journal.ppat.1011449. eCollection 2023 Jun. PLoS Pathog. 2023. PMID: 37352369 Free PMC article.
-
De novo assembly of a field isolate genome reveals novel Plasmodium vivax erythrocyte invasion genes.PLoS Negl Trop Dis. 2013 Dec 5;7(12):e2569. doi: 10.1371/journal.pntd.0002569. eCollection 2013. PLoS Negl Trop Dis. 2013. PMID: 24340114 Free PMC article.
-
The Binding of Plasmodium falciparum Adhesins and Erythrocyte Invasion Proteins to Aldolase Is Enhanced by Phosphorylation.PLoS One. 2016 Sep 8;11(9):e0161850. doi: 10.1371/journal.pone.0161850. eCollection 2016. PLoS One. 2016. PMID: 27607074 Free PMC article.
-
Specific erythrocyte binding capacity and biological activity of Plasmodium falciparum erythrocyte binding ligand 1 (EBL-1)-derived peptides.Protein Sci. 2005 Feb;14(2):464-73. doi: 10.1110/ps.041084305. Protein Sci. 2005. PMID: 15659376 Free PMC article.
-
Erythrocyte invasion by Babesia bovis merozoites is inhibited by polyclonal antisera directed against peptides derived from a homologue of Plasmodium falciparum apical membrane antigen 1.Infect Immun. 2004 May;72(5):2947-55. doi: 10.1128/IAI.72.5.2947-2955.2004. Infect Immun. 2004. PMID: 15102807 Free PMC article.
References
-
- Adams, J.H., P.L. Blair, O. Kaneko, and D.S. Peterson. 2001. An expanding ebl family of Plasmodium falciparum. Trends Parasitol. 17:297–299. - PubMed
-
- Barnwell, J.W., and M.R. Galinski. 1998. Invasion of vertebrate cells: erythrocytes. Malaria: Parasite Biology, Pathogenesis and Protection. I.W. Sherman, editor. ASM Press, Washington, DC. 93–120.

