High-yield expression of isotopically labeled peptides for use in NMR studies
- PMID: 12876327
- PMCID: PMC2323964
- DOI: 10.1110/ps.0376003
High-yield expression of isotopically labeled peptides for use in NMR studies
Abstract
Fusion protein constructs of the 56 amino acid globular protein GB-1 with various peptide sequences, coupled with the incorporation of a histidine tag for affinity purification, have generated high-yield fusion protein constructs. Methionine residues were inserted into the constructs to generate pure peptides following CNBr cleavage, yielding a system that is efficient and cost effective for isotopic labeling of peptides for NMR studies and other disciplines such as mass spectroscopy. Six peptides of varying sequences and hydrophobicities were expressed using this GB-1 fusion protein technique and produced soluble fusion protein constructs in all cases. The ability to easily express and purify recombinant peptides in high yields is applicable for biomedical research and has medicinal and pharmaceutical applications.
Figures


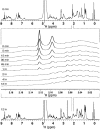

References
-
- Azriel, R. and Gazit, E. 2001. Analysis of the minimal amyloid-forming fragment of the islet amyloid polypeptide. An experimental support for the key role of the phenylalanine residue in amyloid formation. J. Biol. Chem. 276 34156–34161. - PubMed
-
- Campbell, A.P., Van Eyk, J.E., Hodges, R.S., and Sykes, B.D. 1992. Interaction of troponin I and troponin C: Use of the two-dimensional transferred nuclear Overhauser effect to determine the structure of a Gly-110 inhibitory troponin I peptide analog when bound to cardiac troponin C. Biochim. Biophys. Acta 1160 35–54. - PubMed
-
- Counillon, L., Noel, J., Reithmeier, R.A., and Pouyssegur, J. 1997. Random mutagenesis reveals a novel site involved in inhibitor interaction within the fourth transmembrane segment of the Na+/H+ exchanger-1. Biochemistry 36 2951–2959. - PubMed
-
- Delaglio, F., Grzesiek, S., Vuister, G.W., Zhu, G., Pfeifer, J., and Bax, A. 1995. NMRPipe: A multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR 6 277–293. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

