Potent anti-influenza activity of cyanovirin-N and interactions with viral hemagglutinin
- PMID: 12878514
- PMCID: PMC166092
- DOI: 10.1128/AAC.47.8.2518-2525.2003
Potent anti-influenza activity of cyanovirin-N and interactions with viral hemagglutinin
Abstract
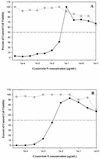
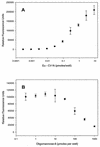
The novel antiviral protein cyanovirin-N (CV-N) was initially discovered based on its potent activity against the human immunodeficiency virus (HIV). Subsequent studies identified the HIV envelope glycoproteins gp120 and gp41 as molecular targets of CV-N. More recently, mechanistic studies have shown that certain high-mannose oligosaccharides (oligomannose-8 and oligomannose-9) found on the HIV envelope glycoproteins comprise the specific sites to which CV-N binds. Such selective, carbohydrate-dependent interactions may account, at least in part, for the unusual and unexpected spectrum of antiviral activity of CV-N described herein. We screened CV-N against a broad range of respiratory and enteric viruses, as well as flaviviruses and herpesviruses. CV-N was inactive against rhinoviruses, human parainfluenza virus, respiratory syncytial virus, and enteric viruses but was moderately active against some herpesvirus and hepatitis virus (bovine viral diarrhea virus) strains (50% effective concentration [EC(50)] = approximately 1 micro g/ml) while inactive against others. Remarkably, however, CV-N and related homologs showed highly potent antiviral activity against almost all strains of influenza A and B virus, including clinical isolates and a neuraminidase inhibitor-resistant strain (EC(50) = 0.004 to 0.04 micro g/ml). When influenza virus particles were pretreated with CV-N, viral titers were lowered significantly (>1,000-fold). Further studies identified influenza virus hemagglutinin as a target for CV-N, showed that antiviral activity and hemagglutinin binding were correlated, and indicated that CV-N's interactions with hemagglutinin involved oligosaccharides. These results further reveal new potential avenues for antiviral therapeutics and prophylaxis targeting specific oligosaccharide-comprised sites on certain enveloped viruses, including HIV, influenza virus, and possibly others.
Figures


References
-
- Arora, D. J. S., P. Tremblay, R. Bourgault, and S. Boileau. 1985. Concentration and purification of influenza virus from allantoic fluid. Anal. Biochem. 144:189-192. - PubMed
-
- Banks, J., E. S. Speidel, E. Moore, L. Plowright, A. Piccirillo, I. Capua, P. Cordioli, A. Fioretti, and D. J. Alexander. 2001. Changes in the haemagglutinin and the neuraminidase genes prior to the emergence of highly pathogenic H7N1 avian influenza viruses in Italy. Arch. Virol. 146:963-973. - PubMed
-
- Barrientos, L. G., B. R. O'Keefe, M. Bray, A. Sanchez, A. M. Gronenborn, and M. R. Boyd. 2002. Cyanovirin-N binds to the viral surface glycoprotein GP1,2 and inhibits infectivity of the Ebola virus. Antivir. Res. 58:47-56. - PubMed
-
- Bewley, C. A. 2001. Solution structure of a cyanovirin-N:Man alpha 1-2Man complex: structural basis for high-affinity carbohydrate-mediated binding of gp120. Structure 9:931-940. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

