Heme binding contributes to antimalarial activity of bis-quaternary ammoniums
- PMID: 12878523
- PMCID: PMC166076
- DOI: 10.1128/AAC.47.8.2584-2589.2003
Heme binding contributes to antimalarial activity of bis-quaternary ammoniums
Erratum in
- Antimicrob Agents Chemother.2003 Oct;47(10):3376
Abstract
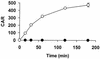
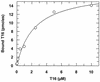
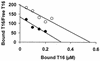
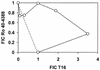
Quaternary ammonium compounds have received recent attention due to their potent in vivo antimalarial activity based on their ability to inhibit de novo phosphatidylcholine synthesis. Here we show that in addition to this, heme binding significantly contributes to the antimalarial activity of these compounds. For the study, we used a recently synthesized bis-quaternary ammonium compound, T16 (1,12-dodecanemethylene bis[4-methyl-5-ethylthiazolium] diodide), which exhibits potent antimalarial activity (50% inhibitory concentration, approximately 25 nM). Accumulation assays reveal that this compound is readily concentrated several hundredfold (cellular accumulation ratio, approximately 500) into parasitized erythrocytes. Approximately 80% of the drug was shown to be distributed within the parasite, approximately 50% of which was located in the parasite food vacuoles. T16 uptake was affected by anion substitution (permeation increasing in the order Cl(-) < Br(-) = NO(3)(-) < I(-) < SCN(-)) and was sensitive to furosemide-properties similar to substrates of the induced new permeability pathway in infected erythrocytes. Scatchard plot analysis of in situ T16 binding revealed high-affinity and low-affinity binding sites. The high-affinity binding site K(d) was similar to that measured in vitro for T16 and ferriprotoporphyrin IX (FPIX) binding. Significantly, the capacity but not the K(d) of the high-affinity binding site was decreased by reducing the concentration of parasite FPIX. Decreasing the parasite FPIX pool also caused a marked antagonism of T16 antimalarial activity. In addition, T16 was also observed to associate with parasite hemozoin. Binding of T16 to FPIX in the digestive food vacuole is shown to be critical for drug accumulation and antimalarial activity. These data provide additional new mechanisms of antimalarial activity for this promising new class of antimalarial compounds.
Figures




References
-
- Ancelin, M. L., M. Calas, J. Bompart, G. Cordina, D. Martin, M. Ben Bari, T. Jei, P. Druilhe, and H. Vial. 1998. Antimalarial activity of 77 phospholipid polar head analogues: close correlation between inhibition of phospholipid metabolism and in vitro Plasmodium falciparum growth. Blood 91:1426-1437. - PubMed
-
- Berenbaum, M. C. 1978. A method for testing for synergy with any number of agents. J. Infect. Dis. 137:122-130. - PubMed
-
- Calas, M., G. Cordina, J. Bompart, M. Ben Bari, T. Jei, M. L. Ancelin, and H. Vial. 1997. Antimalarial activity of molecules interfering with Plasmodium falciparum phospholipid metabolism—structure-activity relationship analysis. J. Med. Chem. 40:3557-3566. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

