Gap junctions mediate bystander cell death in developing retina
- PMID: 12878681
- PMCID: PMC6740641
- DOI: 10.1523/JNEUROSCI.23-16-06413.2003
Gap junctions mediate bystander cell death in developing retina
Abstract
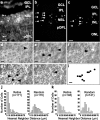
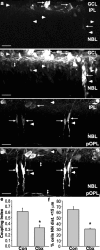
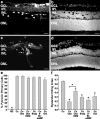
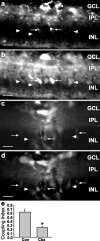
During development of the retina, programmed cell death helps to establish the final size and distribution of various cell classes in distinct layers of the tissue. Here we show that dying cells in the developing ganglion and inner nuclear layers are clustered spatially and that gap junction inhibitors decrease the clustering of dying cells. To confirm the role of gap junctions in cell death, we induced targeted cell death via intracellular cytochrome c (Cc) and examined the induced cells and their neighbors for apoptotic morphology or caspase-3 cleavage. These studies indicate that bystander killing extends to coupled cells. Quantitative studies of bystander killing were performed by scrape-loading retinas with Cc in the presence of rhodamine dextran (RD; to identify Cc-loaded cells) and by counting pyknotic cells in cryosections. Although only 1.5% of control scrape-loaded cells (RD alone) showed apoptotic morphology, 97% of Cc scrape-loaded cells were pyknotic. Moreover, bystander killing extended to neighboring cells, not labeled with RD, and was reduced significantly by the gap junction inhibitors octanol and carbenoxolone. We hypothesize that dying cells in the retina generate a gap junction-permeant apoptotic signal that mediates bystander killing. This novel finding of naturally occurring bystander cell death may have important implications in the histogenesis and pathology of the nervous system.
Figures

References
-
- Becker DL, Bonness V, Catsicas M, Mobbs P ( 2002) Changing patterns of ganglion cell coupling and connexin expression during chick retinal development. J Neurobiol 15: 280-293. - PubMed
-
- Brustugun OT, Fladmark KE, Doskeland SO, Orrenius S, Zhivotovsky B ( 1998) Apoptosis induced by microinjection of cytochrome c is caspase-dependent and is inhibited by Bcl-2. Cell Death Differ 5: 660-668. - PubMed
-
- Chang GQ, Hao Y, Wong F ( 1993) Apoptosis: final common pathway of photoreceptor death in rd, rds, and rhodopsin mutant mice. Neuron 11: 595-605. - PubMed
-
- Cook B, Lewis GP, Fisher SK, Adler R ( 1995) Apoptotic photoreceptor degeneration in experimental retinal detachment. Invest Ophthalmol Vis Sci 36: 990-996. - PubMed
-
- Cook B, Portera-Cailliau C, Adler R ( 1998) Developmental neuronal death is not a universal phenomenon among cell types in the chick embryo retina. J Comp Neurol 396: 12-19. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
