Intermittent hypoxia increases insulin resistance in genetically obese mice
- PMID: 12878760
- PMCID: PMC2343324
- DOI: 10.1113/jphysiol.2003.048173
Intermittent hypoxia increases insulin resistance in genetically obese mice
Abstract
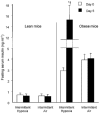
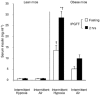
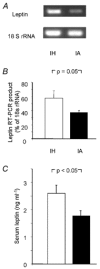
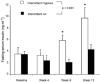
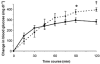
Obstructive sleep apnoea, a syndrome that leads to recurrent intermittent hypoxia, is associated with insulin resistance in obese individuals, but the mechanisms underlying this association remain unknown. We utilized a mouse model to examine the effects of intermittent hypoxia on insulin resistance in lean C57BL/6J mice and leptin-deficient obese (C57BL/6J-Lepob) mice. In lean mice, exposure to intermittent hypoxia for 5 days (short term) resulted in a decrease in fasting blood glucose levels (from 173 +/- 11 mg dl-1 on day 0 to 138 +/- 10 mg dl-1 on day 5, P < 0.01), improvement in glucose tolerance without a change in serum insulin levels and an increase in serum leptin levels in comparison with control (2.6 +/- 0.3 vs. 1.7 +/- 0.2 ng ml-1, P < 0.05). Microarray mRNA analysis of adipose tissue revealed that leptin was the only upregulated gene affecting glucose uptake. In obese mice, short-term intermittent hypoxia led to a decrease in blood glucose levels accompanied by a 607 +/- 136 % (P < 0.01) increase in serum insulin levels. This increase in insulin secretion after 5 days of intermittent hypoxia was completely abolished by prior leptin infusion. Obese mice exposed to intermittent hypoxia for 12 weeks (long term) developed a time-dependent increase in fasting serum insulin levels (from 3.6 +/- 1.1 ng ml-1 at baseline to 9.8 +/- 1.8 ng ml-1 at week 12, P < 0.001) and worsening glucose tolerance, consistent with an increase in insulin resistance. We conclude that the increase in insulin resistance in response to intermittent hypoxia is dependent on the disruption of leptin pathways.
Figures

References
-
- Ambrosini G, Nath AK, Sierra-Honigmann MR, Flores-Riveros J. Transcriptional activation of the human leptin gene in response to hypoxia. Involvement of hypoxia-inducible factor 1. J Biol Chem. 2002;277:34601–34609. - PubMed
-
- Arnalich F, Hernanz A, Lopez-Maderuelo D, Pena JM, Camacho J, Madero R, Vazquez JJ, Montiel C. Enhanced acute-phase response and oxidative stress in older adults with type II diabetes. Horm Metab Res. 2000;32:407–412. - PubMed
-
- Braun B, Rock PB, Zamudio S, Wolfel GE, Mazzeo RS, Muza SR, Fulco CS, Moore LG, Butterfield GE. Women at altitude: short-term exposure to hypoxia and or alpha(1)-adrenergic blockade reduces insulin sensitivity. J Appl Physiol. 2001;91:623–631. - PubMed
-
- Breslow MJ, Min-Lee K, Brown DR, Chacko VP, Palmer D, Berkowitz DE. Effect of leptin deficiency on metabolic rate in ob/ob mice. Am J Physiol. 1999;276:E443–E449. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL071506/HL/NHLBI NIH HHS/United States
- R01 HL037379/HL/NHLBI NIH HHS/United States
- R01 HL 63767/HL/NHLBI NIH HHS/United States
- F32 HL 71469/HL/NHLBI NIH HHS/United States
- R01 HL063767/HL/NHLBI NIH HHS/United States
- K08 HL068715/HL/NHLBI NIH HHS/United States
- K23 HL 04065/HL/NHLBI NIH HHS/United States
- R01 HL 66324/HL/NHLBI NIH HHS/United States
- F32 HL071469/HL/NHLBI NIH HHS/United States
- R01 HL 37379/HL/NHLBI NIH HHS/United States
- R01 HL 71506/HL/NHLBI NIH HHS/United States
- K08 HL 68715/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

