Novel consent process for research in dying patients unable to give consent
- PMID: 12881261
- PMCID: PMC166125
- DOI: 10.1136/bmj.327.7408.198
Novel consent process for research in dying patients unable to give consent
Abstract
Objectives: To develop a process of advance consent to enable research to be undertaken in patients in the terminal phase.
Design: Feasibility study of an advance consent process to support a randomised controlled trial of two antimuscarinic drugs (hyoscine hydrobromide and glycopyrronium bromide) in the management of noisy respirations associated with retained secretions ("death rattle").
Setting: Palliative care wards in a major cancer centre.
Participants: Patients admitted to a palliative care ward who may develop "death rattle" and thus be eligible for randomisation.
Main outcome measures: Patient accrual; acceptability of the consent process.
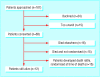
Results: Of the 107 patients approached, 58 patients gave advance consent to participate in the study. Of these, 15 patients developed death rattle and were randomised to receive either hyoscine or glycopyrronium; 16 patients died elsewhere; 15 patients died on the palliative care wards but were not randomised; 12 patients are still alive.
Conclusions: Initial assessment suggests that this is a workable consent process allowing research to be undertaken in patients who are unable to give consent at the time of randomisation. Patient accrual rates to date are lower than needed to recruit adequate numbers in the time allotted to answer the research question.
Similar articles
-
Respiratory tract secretions in the dying patient: a comparison between glycopyrronium and hyoscine hydrobromide.J Palliat Med. 2006 Apr;9(2):279-84. doi: 10.1089/jpm.2006.9.279. J Palliat Med. 2006. PMID: 16629557
-
A study comparing hyoscine hydrobromide and glycopyrrolate in the treatment of death rattle.Palliat Med. 2001 Jul;15(4):329-36. doi: 10.1191/026921601678320313. Palliat Med. 2001. PMID: 12054150 Clinical Trial.
-
Hyoscine and glycopyrrolate for death rattle.Palliat Med. 2002 Sep;16(5):449-50; author reply 450. doi: 10.1191/0269216302pm601xx. Palliat Med. 2002. PMID: 12380666 No abstract available.
-
Hyoscine vs glycopyrronium for drying respiratory secretions in dying patients.Br J Community Nurs. 2005 Sep;10(9):421-4, 426. doi: 10.12968/bjcn.2005.10.9.19688. Br J Community Nurs. 2005. PMID: 16234752 Review.
-
[Reducing loud respiratory sounds of dying patients].Pflege Z. 2014 Oct;67(10):612-3. Pflege Z. 2014. PMID: 25522470 Review. German. No abstract available.
Cited by
-
Patient centred death.BMJ. 2003 Jul 26;327(7408):174-5. doi: 10.1136/bmj.327.7408.174. BMJ. 2003. PMID: 12881232 Free PMC article. No abstract available.
-
Alternative forms of hydration in patients with cancer in the last days of life: study protocol for a randomised controlled trial.Trials. 2015 Oct 14;16:464. doi: 10.1186/s13063-015-0988-3. Trials. 2015. PMID: 26466809 Free PMC article. Clinical Trial.
-
Processes of consent in research for adults with impaired mental capacity nearing the end of life: systematic review and transparent expert consultation (MORECare_Capacity statement).BMC Med. 2020 Jul 22;18(1):221. doi: 10.1186/s12916-020-01654-2. BMC Med. 2020. PMID: 32693800 Free PMC article.
-
Medically assisted hydration for adult palliative care patients.Cochrane Database Syst Rev. 2014 Apr 23;2014(4):CD006273. doi: 10.1002/14651858.CD006273.pub3. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2023 Dec 14;12:CD006273. doi: 10.1002/14651858.CD006273.pub4. PMID: 24760678 Free PMC article. Updated.
-
Medically assisted hydration for adults receiving palliative care.Cochrane Database Syst Rev. 2023 Dec 14;12(12):CD006273. doi: 10.1002/14651858.CD006273.pub4. Cochrane Database Syst Rev. 2023. PMID: 38095590 Free PMC article.
References
-
- Department of Health. www.doh.gov.uk/consent (accessed Nov 2002).
-
- Biros MH, Lewis RJ, Olson CM, Runge JW, Cummins RO, Fost N. Informed consent in emergency research. JAMA 1995;273: 1283-7. - PubMed
-
- Warren JW, Sobal J, Tenney JH, Hoopes JM, Damron D, Levenson S, et al. Informed consent by proxy. N Engl J Med 1986;315: 1124-8. - PubMed
-
- Hardy J. Consent for trials in palliative care. Lancet Perspectives 2000;356: s44. - PubMed
-
- Assessment of mental capacity: guidance for doctors and lawyers: a report of the British Medical Association and the Law Society. London: BMA, 1995.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical