Choroidal neovascular membrane inhibition in a laser treated rat model with intraocular sustained release triamcinolone acetonide microimplants
- PMID: 12881350
- PMCID: PMC1771780
- DOI: 10.1136/bjo.87.8.1032
Choroidal neovascular membrane inhibition in a laser treated rat model with intraocular sustained release triamcinolone acetonide microimplants
Abstract
Aim: To determine if intravitreal microimplants containing triamcinolone acetonide (TAAC) inhibit experimental fibrovascular proliferation (FVP) induced by laser trauma in a rat as a model of choroidal neovascular membranes (CNVMs).
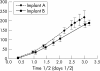
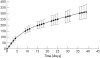
Methods: 20 anaesthetised male Brown Norway rats received a series of eight krypton red laser lesions per eye (647 nm, 0.05 s, 50 micro m, 150 mW). Three types of sterilised TAAC microimplant designs were evaluated: implant A consisting of 8.62% TAAC/20% polyvinyl alcohol (PVA) matrix (by dry weight); implant B consisting of 3.62% TAAC/20% PVA matrix; and implant C consisting of a dual 8.62% TAAC/20% PVA matrix design combined with a central core (0.5 mm) of compressed TAAC to extend the implant release time. For each animal studied, one eye received one of the three aforementioned TAAC implant designs, while the fellow eye received a control implant consisting of PVA but without TAAC. The animals were sacrificed at day 35 and ocular tissues were processed for histological analysis. Serial histological specimens were methodically assessed in a masked fashion to analyse each laser lesion for the presence or absence of FVP; maximum FVP thickness for each lesion was measured from the choriocapillaris.
Results: All three types of TAAC implants inhibited FVP relative to controls in a statistically significant fashion. In the eyes that received implant A (n = 8), the mean thickness of the recovered lesions (n = 36) measured 32 (SD 22) micro m, compared to 52 (30) micro m (p <0.005) for the recovered lesions (n = 40) from the fellow control eyes. In the eyes that received implant B (n = 6), the mean thickness of the recovered lesions (n = 31) measured 28 (15) micro m, compared to 50 (29) micro m (p <0.001) for the lesions (n = 19) recovered from the fellow control eyes. In the eyes that received implant C (n = 6), the mean thickness of the recovered lesions (n = 21) measured 39 (24) micro m, compared to 65 (30) micro m (p <0.001) for the lesions (n = 39) recovered from the fellow control eyes.
Conclusions: All three of the tested TAAC microimplant designs produced potent inhibition of FVP in a rat model of CNVMs. There were no differences in inhibition of FVP between the three different types of implants evaluated. This study provides evidence that: (1) corroborates previous investigations that propose TAAC as a potential treatment for CNVMs in humans, and (2) demonstrates TAAC can be effectively delivered via long acting sustained release intraocular microimplants. It should be noted, however, that the FVP observed in this rat laser trauma may not reflect the CNVM observed in human with exudative age related macular degeneration (AMD).
Figures


Similar articles
-
Intravitreal triamcinolone acetonide inhibits choroidal neovascularization in a laser-treated rat model.Arch Ophthalmol. 2001 Mar;119(3):399-404. doi: 10.1001/archopht.119.3.399. Arch Ophthalmol. 2001. PMID: 11231773
-
Influence of triamcinolone intravitreal injection on retinochoroidal healing processes.Exp Eye Res. 2007 Jun;84(6):1081-9. doi: 10.1016/j.exer.2007.01.024. Epub 2007 Feb 11. Exp Eye Res. 2007. PMID: 17408616
-
Squalamine lactate reduces choroidal neovascularization in a laser-injury model in the rat.Retina. 2003 Dec;23(6):808-14. doi: 10.1097/00006982-200312000-00011. Retina. 2003. PMID: 14707832
-
[Intravitreal triamcinolone acetonide for the treatment of intraocular edematous and neovascular diseases].Ophthalmologe. 2004 Feb;101(2):113-20. doi: 10.1007/s00347-003-0982-0. Ophthalmologe. 2004. PMID: 14991306 Review. German.
-
Intravitreal triamcinolone acetonide for treatment of intraocular oedematous and neovascular diseases.Acta Ophthalmol Scand. 2005 Dec;83(6):645-63. doi: 10.1111/j.1600-0420.2005.00592.x. Acta Ophthalmol Scand. 2005. PMID: 16396641 Review.
Cited by
-
Photodynamic therapy following intravitreal bevacizumab in multifocal choroiditis.Int Ophthalmol. 2008 Oct;28(5):375-7. doi: 10.1007/s10792-007-9146-6. Epub 2007 Oct 3. Int Ophthalmol. 2008. PMID: 17912486
-
Intravitreal Topotecan Inhibits Laser-induced Choroidal Neovascularization in a Rat Model.J Ophthalmic Vis Res. 2015 Jul-Sep;10(3):295-302. doi: 10.4103/2008-322X.170339. J Ophthalmic Vis Res. 2015. PMID: 26730316 Free PMC article.
-
Intraocular sustained-release delivery systems for triamcinolone acetonide.Pharm Res. 2009 Apr;26(4):770-84. doi: 10.1007/s11095-008-9812-z. Epub 2009 Jan 28. Pharm Res. 2009. PMID: 19184374 Review.
-
Incomplete response to Anti-VEGF therapy in neovascular AMD: Exploring disease mechanisms and therapeutic opportunities.Prog Retin Eye Res. 2021 May;82:100906. doi: 10.1016/j.preteyeres.2020.100906. Epub 2020 Oct 3. Prog Retin Eye Res. 2021. PMID: 33022379 Free PMC article. Review.
-
Combined photodynamic therapy and intravitreal triamcinolone injection for the treatment of subfoveal choroidal neovascularisation in age related macular degeneration: a comparative study.Br J Ophthalmol. 2006 Mar;90(3):337-41. doi: 10.1136/bjo.2005.081299. Br J Ophthalmol. 2006. PMID: 16488958 Free PMC article. Clinical Trial.
References
-
- Penfold P, Gyory J, Hunyor A, et al. Exudative macular degeneration and intravitreal triamcinolone. A pilot study. Aust N Z J Ophthalmol 1995;23:293–8. - PubMed
-
- Challa JK, Gillies MC, Penfold PL, et al. Exudative macular degeneration and intravitreal triamcinolone:18 month follow up. Aust N Z J Ophthalmol 1998;26:277–81. - PubMed
-
- Danis RP, Ciulla TA, Pratt LM, et al. Intravitreal triamcinolone acetonide in exudative age-related macular degeneration. Retina 2000;20:244–50. - PubMed
-
- Tobe T, Takahashi K, Ohkuma HU, et al. Experimental choroidal neovascularization in the rat. Nippon Ganka Gakkai Zashi 1994;98:837–45. - PubMed
-
- Dobi E, Puliafito C, Destro M. A new model of experimental choroidal neovascularization in the rat. Arch Ophthalmol 1989;107:264–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
