The guanylate binding protein-1 GTPase controls the invasive and angiogenic capability of endothelial cells through inhibition of MMP-1 expression
- PMID: 12881412
- PMCID: PMC169055
- DOI: 10.1093/emboj/cdg382
The guanylate binding protein-1 GTPase controls the invasive and angiogenic capability of endothelial cells through inhibition of MMP-1 expression
Abstract
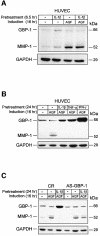
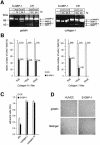
Expression of the large GTPase guanylate binding protein-1 (GBP-1) is induced by inflammatory cytokines (ICs) in endothelial cells (ECs), and the helical domain of the molecule mediates the repression of EC proliferation by ICs. Here we show that the expression of GBP-1 and of the matrix metalloproteinase-1 (MMP-1) are inversely related in vitro and in vivo, and that GBP-1 selectively inhibits the expression of MMP-1 in ECs, but not the expression of other proteases. The GTPase activity of GBP-1 was necessary for this effect, which inhibited invasiveness and tube-forming capability of ECs in three-dimensional collagen-I matrices. A GTPase-deficient mutant (D184N-GBP-1) operated as a transdominant inhibitor of wild-type GBP-1 and rescued MMP-1 expression in the presence of ICs. Expression of D184N-GBP-1, as well as paracrine supplementation of MMP-1, restored the tube-forming capability of ECs in the presence of wild-type GBP-1. The latter finding indicated that the inhibition of capillary formation is specifically due to the repression of MMP-1 expression by GBP-1, and is not affected by the anti-proliferative activity of the helical domain of GBP-1. These findings substantiate the role of GBP-1 as a major regulator of the anti-angiogenic response of ECs to ICs.
Figures




Similar articles
-
The helical domain of GBP-1 mediates the inhibition of endothelial cell proliferation by inflammatory cytokines.EMBO J. 2001 Oct 15;20(20):5568-77. doi: 10.1093/emboj/20.20.5568. EMBO J. 2001. PMID: 11598000 Free PMC article.
-
Nuclear factor-kappaB motif and interferon-alpha-stimulated response element co-operate in the activation of guanylate-binding protein-1 expression by inflammatory cytokines in endothelial cells.Biochem J. 2004 Apr 15;379(Pt 2):409-20. doi: 10.1042/BJ20031873. Biochem J. 2004. PMID: 14741045 Free PMC article.
-
MMP-1 activation by serine proteases and MMP-10 induces human capillary tubular network collapse and regression in 3D collagen matrices.J Cell Sci. 2005 May 15;118(Pt 10):2325-40. doi: 10.1242/jcs.02360. Epub 2005 May 3. J Cell Sci. 2005. PMID: 15870107
-
Human guanylate binding protein-1 (hGBP-1) characterizes and establishes a non-angiogenic endothelial cell activation phenotype in inflammatory diseases.Adv Enzyme Regul. 2005;45:215-27. doi: 10.1016/j.advenzreg.2005.02.011. Epub 2005 Jul 6. Adv Enzyme Regul. 2005. PMID: 16005050 Review.
-
Pathophysiological role of guanylate-binding proteins in gastrointestinal diseases.World J Gastroenterol. 2016 Jul 28;22(28):6434-43. doi: 10.3748/wjg.v22.i28.6434. World J Gastroenterol. 2016. PMID: 27605879 Free PMC article. Review.
Cited by
-
Suppressor of cytokine signalling protein SOCS1 and UBP43 regulate the expression of type I interferon-stimulated genes in human microvascular endothelial cells infected with Rickettsia conorii.J Med Microbiol. 2013 Jul;62(Pt 7):968-979. doi: 10.1099/jmm.0.054502-0. Epub 2013 Apr 4. J Med Microbiol. 2013. PMID: 23558133 Free PMC article.
-
The interferon-gamma-induced murine guanylate-binding protein-2 inhibits rac activation during cell spreading on fibronectin and after platelet-derived growth factor treatment: role for phosphatidylinositol 3-kinase.Mol Biol Cell. 2010 Jul 15;21(14):2514-28. doi: 10.1091/mbc.e09-04-0344. Epub 2010 May 26. Mol Biol Cell. 2010. PMID: 20505078 Free PMC article.
-
The clinical value of von Willebrand factor in colorectal carcinomas.Am J Transl Res. 2011;3(5):445-53. Epub 2011 Sep 23. Am J Transl Res. 2011. PMID: 22046486 Free PMC article.
-
Capturing resilience from phenotypic deviations: a case study using feed consumption and whole genome data in pigs.BMC Genomics. 2024 Nov 21;25(1):1128. doi: 10.1186/s12864-024-11052-0. BMC Genomics. 2024. PMID: 39574040 Free PMC article.
-
Guanylate-binding protein 1 expression from embryonal endothelial progenitor cells reduces blood vessel density and cellular apoptosis in an axially vascularised tissue-engineered construct.BMC Biotechnol. 2012 Dec 6;12:94. doi: 10.1186/1472-6750-12-94. BMC Biotechnol. 2012. PMID: 23217187 Free PMC article.
References
-
- Benbow U., Tower,G.B., Wyatt,C.A., Buttice,G. and Brinckerhoff,C.E. (2002) High levels of MMP-1 expression in the absence of the 2G single nucleotide polymorphism is mediated by p38 and ERK1/2 mitogen-activated protein kinases in VMM5 melanoma cells. J. Cell. Biochem., 86, 307–319. - PubMed
-
- Cardozo A.K., Heimberg,H., Heremans,Y., Leeman,R., Kutlu,B., Kruhoffer,M., Orntoft,T. and Eizirik,D.L. (2001) A comprehensive analysis of cytokine-induced and nuclear factor-κB-dependent genes in primary rat pancreatic beta-cells. J. Biol. Chem., 276, 48879–48886. - PubMed
-
- Carmeliet P. and Jain,R.K. (2000) Angiogenesis in cancer and other diseases. Nature, 407, 249–257. - PubMed
-
- Cheng Y.S., Colonno,R.J. and Yin,F.H. (1983) Interferon induction of fibroblast proteins with guanylate binding activity. J. Biol. Chem., 258, 7746–7750. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

