Structure of a specific alcohol-binding site defined by the odorant binding protein LUSH from Drosophila melanogaster
- PMID: 12881720
- PMCID: PMC4397894
- DOI: 10.1038/nsb960
Structure of a specific alcohol-binding site defined by the odorant binding protein LUSH from Drosophila melanogaster
Erratum in
- Nat Struct Biol. 2004 Jan;11(1):102
Abstract
We have solved the high-resolution crystal structures of the Drosophila melanogaster alcohol-binding protein LUSH in complex with a series of short-chain n-alcohols. LUSH is the first known nonenzyme protein with a defined in vivo alcohol-binding function. The structure of LUSH reveals a set of molecular interactions that define a specific alcohol-binding site. A group of amino acids, Thr57, Ser52 and Thr48, form a network of concerted hydrogen bonds between the protein and the alcohol that provides a structural motif to increase alcohol-binding affinity at this site. This motif seems to be conserved in a number of mammalian ligand-gated ion channels that are directly implicated in the pharmacological effects of alcohol. Further, these sequences are found in regions of ion channels that are known to confer alcohol sensitivity. We suggest that the alcohol-binding site in LUSH represents a general model for alcohol-binding sites in proteins.
Figures
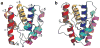

References
-
- Faingold CL, N’Gouemo P, Riaz A. Ethanol and neurotransmitter interactions - From molecular to integrative effects. Prog Neurobiol. 1998;55:509–535. - PubMed
-
- Ikonomidou C, et al. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science. 2000;287:1056–1060. - PubMed
-
- Lovinger DM. Alcohols and neurotransmitter gated ion channels: Past, present and future. Naunyn-Schmiedebergs Arch Pharmacol. 1997;356:267–282. - PubMed
-
- Harris RA. Ethanol actions on multiple ion channels: Which are important? Alcoholism (NY) 1999;23:1563–1570. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
