A constitutively activated mutant of human soluble guanylyl cyclase (sGC): implication for the mechanism of sGC activation
- PMID: 12883009
- PMCID: PMC170897
- DOI: 10.1073/pnas.1633590100
A constitutively activated mutant of human soluble guanylyl cyclase (sGC): implication for the mechanism of sGC activation
Abstract
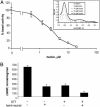
Heterodimeric alphabeta soluble guanylyl cyclase (sGC) is a recognized receptor for nitric oxide (NO) and mediates many of its physiological functions. Although it has been clear that the heme moiety coordinated by His-105 of the beta subunit is crucial for mediating the activation of the enzyme by NO, it is not understood whether the heme moiety plays any role in the function of the enzyme in the absence of NO. Here we analyze the effects of biochemical and genetic removal of heme and its reconstitution on the activity of the enzyme. Detergent-induced loss of heme from the wild-type alphabeta enzyme resulted in several-fold activation of the enzyme. This activation was inhibited after hemin reconstitution. A heme-deficient mutant alphabetaCys-105 with Cys substituted for His-105 was constitutively active with specific activity approaching the activity of the wild-type enzyme activated by NO. However, reconstitution of mutant enzyme with heme and/or DTT treatment significantly inhibited the enzyme. Mutant enzyme reconstituted with ferrous heme was activated by NO and CO alone and showed additive effects between gaseous effectors and the allosteric activator 5-cyclopropyl-2-[1-(2-fluoro-benzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl]-pyrimidin-4-ylamine. We propose that the heme moiety through its coordination with His-105 of the beta subunit acts as an endogenous inhibitor of sGC. Disruption of the heme-coordinating bond induced by binding of NO releases the restrictions imposed by this bond and allows the formation of an optimally organized catalytic center in the heterodimer.
Figures




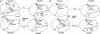
Similar articles
-
Distinct molecular requirements for activation or stabilization of soluble guanylyl cyclase upon haem oxidation-induced degradation.Br J Pharmacol. 2009 Jul;157(5):781-95. doi: 10.1111/j.1476-5381.2009.00263.x. Epub 2009 May 18. Br J Pharmacol. 2009. PMID: 19466990 Free PMC article.
-
Nitric oxide sensitive guanylyl cyclase activity decreases during cerebral postnatal development because of a reduction in heterodimerization.J Neurochem. 2010 Jan;112(2):542-51. doi: 10.1111/j.1471-4159.2009.06484.x. Epub 2009 Nov 6. J Neurochem. 2010. PMID: 19895661
-
On the activation of soluble guanylyl cyclase by nitric oxide.Proc Natl Acad Sci U S A. 2002 Jan 8;99(1):507-10. doi: 10.1073/pnas.012368499. Epub 2001 Dec 18. Proc Natl Acad Sci U S A. 2002. PMID: 11752394 Free PMC article.
-
Structure and Activation of Soluble Guanylyl Cyclase, the Nitric Oxide Sensor.Antioxid Redox Signal. 2017 Jan 20;26(3):107-121. doi: 10.1089/ars.2016.6693. Epub 2016 Apr 26. Antioxid Redox Signal. 2017. PMID: 26979942 Free PMC article. Review.
-
Regulation of nitric oxide and soluble guanylyl cyclase.Brain Res Bull. 2004 Feb 15;62(6):505-15. doi: 10.1016/S0361-9230(03)00102-3. Brain Res Bull. 2004. PMID: 15036565 Review.
Cited by
-
Role of nitric oxide and cGMP in the modulation of vascular contraction induced by angiotensin II and Bay K8644 during ischemia/reperfusion.Exp Ther Med. 2013 Feb;5(2):616-620. doi: 10.3892/etm.2012.846. Epub 2012 Nov 30. Exp Ther Med. 2013. PMID: 23407814 Free PMC article.
-
Inactivation of soluble guanylyl cyclase in living cells proceeds without loss of haem and involves heterodimer dissociation as a common step.Br J Pharmacol. 2022 Jun;179(11):2505-2518. doi: 10.1111/bph.15527. Epub 2021 Jun 16. Br J Pharmacol. 2022. PMID: 33975383 Free PMC article.
-
Constitutive cyclic GMP accumulation in Arabidopsis thaliana compromises systemic acquired resistance induced by an avirulent pathogen by modulating local signals.Sci Rep. 2016 Nov 4;6:36423. doi: 10.1038/srep36423. Sci Rep. 2016. PMID: 27811978 Free PMC article.
-
Dynamic ligand exchange in soluble guanylyl cyclase (sGC): implications for sGC regulation and desensitization.J Biol Chem. 2011 Dec 16;286(50):43182-92. doi: 10.1074/jbc.M111.290304. Epub 2011 Oct 18. J Biol Chem. 2011. PMID: 22009742 Free PMC article.
-
Restoring soluble guanylyl cyclase expression and function blocks the aggressive course of glioma.Mol Pharmacol. 2011 Dec;80(6):1076-84. doi: 10.1124/mol.111.073585. Epub 2011 Sep 9. Mol Pharmacol. 2011. PMID: 21908708 Free PMC article.
References
-
- Rodgers, K. R. (1999) Curr. Opin. Chem. Biol. 3, 158–167. - PubMed
-
- Murad, F. (1998) Recent Prog. Horm. Res. 53, 43–59. - PubMed
-
- Pellequer, J. L., Brudler, R. & Getzoff, E. D. (1999) Curr. Biol. 9, R416–R418. - PubMed
-
- Aono, S., Honma, Y., Ohkubo, K., Tawara, T., Kamiya, T. & Nakajima, H. (2000) J. Inorg. Biochem. 82, 51–56. - PubMed
-
- Lucas, K. A., Pitari, G. M., Kazerounian, S., Ruiz-Stewart, I., Park, J., Schulz, S., Chepenik, K. P. & Waldman, S. A. (2000) Pharmacol. Rev. 52, 375–414. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

