Solvation of nucleosides in aqueous mixtures of organic solvents: relevance to DNA open basepairs
- PMID: 12885656
- PMCID: PMC1303230
- DOI: 10.1016/S0006-3495(03)74548-2
Solvation of nucleosides in aqueous mixtures of organic solvents: relevance to DNA open basepairs
Abstract
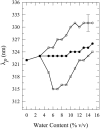
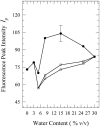
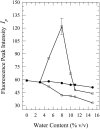
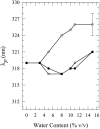
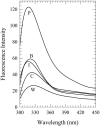
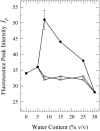
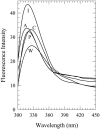
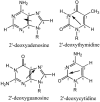
Toward the goal of understanding how open basepairs in DNA interact with their heterogeneous environment, we have studied the steady-state intrinsic fluorescence properties of the purine and pyrimidine deoxynucleosides in organic solvents in the presence of small amounts of water. The organic solvents used in the present study were: n-butanol, acetonitrile, methanol, n-propanol, isopropanol, and isobutanol. For n-butanol and acetonitrile, which have a high degree of amphiphilicity and weak hydrogen bonding ability, respectively, the fluorescence spectral properties of the purines are found to depend on the sequence of steps in which the aqueous mixtures were formed. By contrast, no such dependence was observed in the mixtures with any of the other solvents used in the present study. Moreover, no such dependence was observed for the pyrimidines. These findings suggest that the final solvation network around the purines is dependent on the nature of the environment to which they were initially exposed. This would tend to present an impediment to the closing of AT or GC basepairs in DNA that become open as a result of structural fluctuations, DNA bending, or protein-DNA interactions.
Figures




References
-
- Alyoubi, A. O., and R. H. Hilal. 1995. A theoretical and experimental investigation of the electronic spectra and tautomerism of nucleobases. Biophys. Chem. 55:231–237. - PubMed
-
- Bergman, D. L., and A. Laaksonen. 1998. Topological and spatial structure in the liquid water-acetonitrile mixture. Phys. Rev. E. 58:4706–4715.
-
- Berman, H. M. 1994. Hydration of DNA: take 2. Curr. Opin. Struct. Biol. 4:345–350.
-
- Bernet, J., K. Zakrzewska, and R. Lavery. 1997. Modeling base pair opening: the role of helical twist. J. Mol. Struct. 398–399:473–482.
-
- Bertie, J. E., and Z. Lan. 1997. Liquid water-acetonitrile mixtures at 25°C: the hydrogen-bonded structure studied through infrared absolute integrated absorption intensities. J. Phys. Chem. B. 101:4111–4119.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

