Regulation of cell death in mitotic neural progenitor cells by asymmetric distribution of prostate apoptosis response 4 (PAR-4) and simultaneous elevation of endogenous ceramide
- PMID: 12885759
- PMCID: PMC2172704
- DOI: 10.1083/jcb.200212067
Regulation of cell death in mitotic neural progenitor cells by asymmetric distribution of prostate apoptosis response 4 (PAR-4) and simultaneous elevation of endogenous ceramide
Abstract
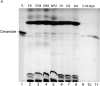
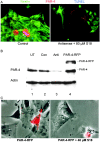
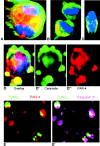
Cell death and survival of neural progenitor (NP) cells are determined by signals that are largely unknown. We have analyzed pro-apoptotic signaling in individual NP cells that have been derived from mouse embryonic stem cells. NP formation was concomitant with elevated apoptosis and increased expression of ceramide and prostate apoptosis response 4 (PAR-4). Morpholino oligonucleotide-mediated antisense knockdown of PAR-4 or inhibition of ceramide biosynthesis reduced stem cell apoptosis, whereas PAR-4 overexpression and treatment with ceramide analogs elevated apoptosis. Apoptotic cells also stained for proliferating cell nuclear antigen (a nuclear mitosis marker protein), but not for nestin (a marker for NP cells). In mitotic cells, asymmetric distribution of PAR-4 and nestin resulted in one nestin(-)/PAR-4(+) daughter cell, in which ceramide elevation induced apoptosis. The other cell was nestin(+), but PAR-4(-), and was not apoptotic. Asymmetric distribution of PAR-4 and simultaneous elevation of endogenous ceramide provides a possible mechanism underlying asymmetric differentiation and apoptosis of neuronal stem cells in the developing brain.
Figures










References
-
- Bieberich, E., S. MacKinnon, J. Silva, and R.K. Yu. 2001. Regulation of apoptosis during neuronal differentiation by ceramide and b-series complex gangliosides. J. Biol. Chem. 276:44396–44404. - PubMed
-
- Blaschke, A.J., K. Staley, and J. Chun. 1996. Widespread programmed cell death in proliferative and post-mitotic regions of fetal cerebral cortex. Development. 122:1165–1174. - PubMed
-
- Camandola, S., and M.P. Mattson. 2000. Pro-apoptotic action of Par-4 involves inhibition of NF-κB activity and suppression of bcl-2 expression. J. Neurosci. Res. 61:134–139. - PubMed
-
- Cecconi, F., G. Alvarez-Bolado, B.I. Meyer, K.A. Roth, and P. Gruss. 1998. Apaf1 (CED-4 homolog) regulates programmed cell death in mammalian development. Cell. 94:727–737. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

