The ER v-SNAREs are required for GPI-anchored protein sorting from other secretory proteins upon exit from the ER
- PMID: 12885760
- PMCID: PMC2172695
- DOI: 10.1083/jcb.200212101
The ER v-SNAREs are required for GPI-anchored protein sorting from other secretory proteins upon exit from the ER
Abstract
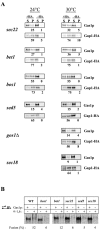
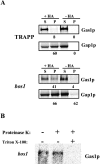
Glycosylphosphatidylinositol (GPI)-anchored proteins exit the ER in distinct vesicles from other secretory proteins, and this sorting event requires the Rab GTPase Ypt1p, tethering factors Uso1p, and the conserved oligomeric Golgi complex. Here we show that proper sorting depended on the vSNAREs, Bos1p, Bet1p, and Sec22p. However, the t-SNARE Sed5p was not required for protein sorting upon ER exit. Moreover, the sorting defect observed in vitro with bos1-1 extracts was also observed in vivo and was visualized by EM. Finally, transport and maturation of the GPI-anchored protein Gas1p was specifically affected in a bos1-1 mutant at semirestrictive temperature. Therefore, we propose that v-SNAREs are part of the cargo protein sorting machinery upon exit from the ER and that a correct sorting process is necessary for proper maturation of GPI-anchored proteins.
Figures




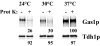
References
-
- Allan, B.B., B.D. Moyer, and W.E. Balch. 2000. Rab1 recruitment of p115 into a cis-SNARE complex: programming budding COPII vesicles for fusion. Science. 289:444–448. - PubMed
-
- Carroll, K.S., J. Hanna, I. Simon, J. Krise, P. Barbero, and S.R. Pfeffer. 2001. Role of rab9 GTPase in facilitating receptor recruitment by tip47. Science. 292:1373–1376. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

