Importin beta contains a COOH-terminal nucleoporin binding region important for nuclear transport
- PMID: 12885761
- PMCID: PMC2172684
- DOI: 10.1083/jcb.200303085
Importin beta contains a COOH-terminal nucleoporin binding region important for nuclear transport
Abstract
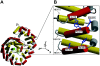
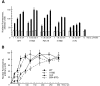
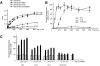
Proteins containing a classical NLS are transported into the nucleus by the import receptor importin beta, which binds to cargoes via the adaptor importin alpha. The import complex is translocated through the nuclear pore complex by interactions of importin beta with a series of nucleoporins. Previous studies have defined a nucleoporin binding region in the NH2-terminal half of importin beta. Here we report the identification of a second nucleoporin binding region in its COOH-terminal half. Although the affinity of the COOH-terminal region for nucleoporins is dramatically weaker than that of the NH2-terminal region, sets of mutations that perturb the nucleoporin binding of either region reduce the nuclear import activity of importin beta to a similar extent ( approximately 50%). An importin beta mutant with a combination of mutations in the NH2- and COOH-terminal regions is completely inactive for nuclear import. Thus, importin beta possesses two nucleoporin binding sites, both of which are important for its nuclear import function.
Figures

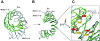
References
-
- Adam, S.A., R. Sterne-Marr, and L. Gerace. 1992. Nuclear protein import using digitonin-permeabilized cells. Methods Enzymol. 219:97–110. - PubMed
-
- Bayliss, R., K. Ribbeck, D. Akin, H.M. Kent, C.M. Feldherr, D. Görlich, and M. Stewart. 1999. Interaction between NTF2 and xFxFG-containing nucleoporins is required to mediate nuclear import of RanGDP. J. Mol. Biol. 293:579–593. - PubMed
-
- Bayliss, R., T. Littlewood, and M. Stewart. 2000. Structural basis for the interaction between FxFG nucleoporin repeats and importin β in nuclear trafficking. Cell. 102:99–108. - PubMed
-
- Bayliss, R., T. Littlewood, L.A. Strawn, S.R. Wente, and M. Stewart. 2002. GLFG and FxFG nucleoporins bind to overlapping sites on importin β. J. Biol. Chem. 277:50597–50606. - PubMed
-
- Bednenko, J., G. Cingolani, and L. Gerace. 2003. Nucleocytoplasmic transport: navigating the channel. Traffic. 4:127–135. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

