Differential antigen presentation regulates the changing patterns of CD8+ T cell immunodominance in primary and secondary influenza virus infections
- PMID: 12885871
- PMCID: PMC2194086
- DOI: 10.1084/jem.20022151
Differential antigen presentation regulates the changing patterns of CD8+ T cell immunodominance in primary and secondary influenza virus infections
Abstract
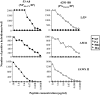
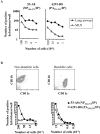
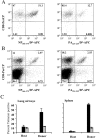
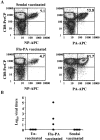
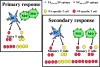
The specificity of CD8+ T cell responses can vary dramatically between primary and secondary infections. For example, NP366-374/Db- and PA224-233/Db-specific CD8+ T cells respond in approximately equal numbers to a primary influenza virus infection in C57BL/6 mice, whereas NP366-374/Db-specific CD8+ T cells dominate the secondary response. To investigate the mechanisms underlying this changing pattern of immunodominance, we analyzed the role of antigen presentation in regulating the specificity of the T cell response. The data show that both dendritic and nondendritic cells are able to present the NP366-374/Db epitope, whereas only dendritic cells effectively present the PA224-233/Db epitope after influenza virus infection, both in vitro and in vivo. This difference in epitope expression favored the activation and expansion of NP366-374/Db-specific CD8+ memory T cells during secondary infection. The data also show that the immune response to influenza virus infection may involve T cells specific for epitopes, such as PA224-233/Db, that are poorly expressed at the site of infection. In this regard, vaccination with the PA224-233 peptide actually had a detrimental effect on the clearance of a subsequent influenza virus infection. Thus, differential antigen presentation impacts both the specificity of the T cell response and the efficacy of peptide-based vaccination strategies.
Figures



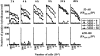

References
-
- Kast, W.M., L. Roux, J. Curren, H.J. Blom, A.C. Voordouw, R.H. Meloen, D. Kolakofsky, and C.J. Melief. 1991. Protection against lethal Sendai virus infection by in vivo priming of virus-specific cytotoxic T lymphocytes with a free synthetic peptide. Proc. Natl. Acad. Sci. USA. 88:2283–2287. - PMC - PubMed
-
- Cole, G.A., T.L. Hogg, and D.L. Woodland. 1994. The MHC class I-restricted T cell response to Sendai virus infection in C57BL/6 mice: a single immunodominant epitope elicits an extremely diverse repertoire of T cells. Int. Immunol. 6:1767–1775. - PubMed
-
- Yewdell, J.W., and J.R. Bennink. 1999. Immunodominance in major histocompatibility complex class I-restricted T lymphocyte responses. Annu. Rev. Immunol. 17:51–88. - PubMed
-
- Chen, W., L.C. Anton, J.R. Bennink, and J.W. Yewdell. 2000. Dissecting the multifactorial causes of immunodominance in class I-restricted T cell responses to viruses. Immunity. 12:83–93. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

