Regulation of cyclooxygenase-2 expression by the translational silencer TIA-1
- PMID: 12885872
- PMCID: PMC2194089
- DOI: 10.1084/jem.20030616
Regulation of cyclooxygenase-2 expression by the translational silencer TIA-1
Abstract
The cyclooxygenase-2 (COX-2) enzyme catalyzes the rate-limiting step of prostaglandin formation in inflammatory states, and COX-2 overexpression plays a key role in carcinogenesis. To understand the mechanisms regulating COX-2 expression, we examined its posttranscriptional regulation mediated through the AU-rich element (ARE) within the COX-2 mRNA 3'-untranslated region (3'UTR). RNA binding studies, performed to identify ARE-binding regulatory factors, demonstrated binding of the translational repressor protein TIA-1 to COX-2 mRNA. The significance of TIA-1-mediated regulation of COX-2 expression was observed in TIA-1 null fibroblasts that produced significantly more COX-2 protein than wild-type fibroblasts. However, TIA-1 deficiency did not alter COX-2 transcription or mRNA turnover. Colon cancer cells demonstrated to overexpress COX-2 through increased polysome association with COX-2 mRNA also showed defective TIA-1 binding both in vitro and in vivo. These findings implicate that TIA-1 functions as a translational silencer of COX-2 expression and support the hypothesis that dysregulated RNA-binding of TIA-1 promotes COX-2 expression in neoplasia.
Figures
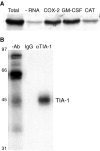


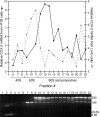

References
-
- Gupta, R.A., and R.N. Dubois. 2001. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat. Rev. Cancer. 1:11–21. - PubMed
-
- Dixon, D.A. 2003. Regulation of COX-2 expression in human cancer. Prog. Exp. Tumor Res. 37:70–89. - PubMed
-
- Dixon, D.A., C.D. Kaplan, T.M. McIntyre, G.A. Zimmerman, and S.M. Prescott. 2000. Post-transcriptional control of cyclooxygenase-2 gene expression. The role of the 3′-untranslated region. J. Biol. Chem. 275:11750–11757. - PubMed
-
- Sheng, H., J. Shao, D.A. Dixon, C.S. Williams, S.M. Prescott, R.N. DuBois, and R.D. Beauchamp. 2000. Transforming growth factor-beta1 enhances Ha-ras-induced expression of cyclooxygenase-2 in intestinal epithelial cells via stabilization of mRNA. J. Biol. Chem. 275:6628–6635. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI50167/AI/NIAID NIH HHS/United States
- R56 AI033600/AI/NIAID NIH HHS/United States
- R01 AI033600/AI/NIAID NIH HHS/United States
- P30 CA68485/CA/NCI NIH HHS/United States
- P01 CA73992/CA/NCI NIH HHS/United States
- CA42014/CA/NCI NIH HHS/United States
- AI33600/AI/NIAID NIH HHS/United States
- P01 CA77839/CA/NCI NIH HHS/United States
- P01 CA077839/CA/NCI NIH HHS/United States
- R01 AI050167/AI/NIAID NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- DK-52334/DK/NIDDK NIH HHS/United States
- P01 CA073992/CA/NCI NIH HHS/United States
- R01 DK052334/DK/NIDDK NIH HHS/United States
- P30 CA042014/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

