Stabilization of the activity of ATP-sensitive potassium channels by ion pairs formed between adjacent Kir6.2 subunits
- PMID: 12885877
- PMCID: PMC2229541
- DOI: 10.1085/jgp.200308822
Stabilization of the activity of ATP-sensitive potassium channels by ion pairs formed between adjacent Kir6.2 subunits
Abstract
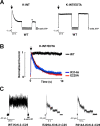
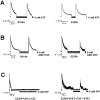
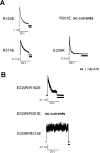
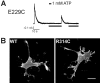
ATP-sensitive potassium (KATP) channels are formed by the coassembly of four Kir6.2 subunits and four sulfonylurea receptor subunits (SUR). The cytoplasmic domains of Kir6.2 mediate channel gating by ATP, which closes the channel, and membrane phosphoinositides, which stabilize the open channel. Little is known, however, about the tertiary or quaternary structures of the domains that are responsible for these interactions. Here, we report that an ion pair between glutamate 229 and arginine 314 in the intracellular COOH terminus of Kir6.2 is critical for maintaining channel activity. Mutation of either residue to alanine induces inactivation, whereas charge reversal at positions 229 and 314 (E229R/R314E) abolishes inactivation and restores the wild-type channel phenotype. The close proximity of these two residues is demonstrated by disulfide bond formation between cysteine residues introduced at the two positions (E229C/R314C); disulfide bond formation abolishes inactivation and stabilizes the current. Using Kir6.2 tandem dimer constructs, we provide evidence that the ion pair likely forms by residues from two adjacent Kir6.2 subunits. We propose that the E229/R314 intersubunit ion pairs may contribute to a structural framework that facilitates the ability of other positively charged residues to interact with membrane phosphoinositides. Glutamate and arginine residues are found at homologous positions in many inward rectifier subunits, including the G-protein-activated inwardly rectifying potassium channel (GIRK), whose cytoplasmic domain structure has recently been solved. In the GIRK structure, the E229- and R314-corresponding residues are oriented in opposite directions in a single subunit such that in the tetramer model, the E229 equivalent residue from one subunit is in close proximity of the R314 equivalent residue from the adjacent subunit. The structure lends support to our findings in Kir6.2, and raises the possibility that a homologous ion pair may be involved in the gating of GIRKs.
Figures


Similar articles
-
Conformational dynamics of the ligand-binding domain of inward rectifier K channels as revealed by molecular dynamics simulations: toward an understanding of Kir channel gating.Biophys J. 2005 May;88(5):3310-20. doi: 10.1529/biophysj.104.052019. Epub 2005 Mar 4. Biophys J. 2005. PMID: 15749783 Free PMC article.
-
Destabilization of ATP-sensitive potassium channel activity by novel KCNJ11 mutations identified in congenital hyperinsulinism.J Biol Chem. 2008 Apr 4;283(14):9146-56. doi: 10.1074/jbc.M708798200. Epub 2008 Feb 4. J Biol Chem. 2008. PMID: 18250167 Free PMC article.
-
ATP sensitivity of ATP-sensitive K+ channels: role of the gamma phosphate group of ATP and the R50 residue of mouse Kir6.2.J Physiol. 2005 Nov 1;568(Pt 3):931-40. doi: 10.1113/jphysiol.2005.095638. Epub 2005 Sep 15. J Physiol. 2005. PMID: 16166157 Free PMC article.
-
Molecular biology of adenosine triphosphate-sensitive potassium channels.Endocr Rev. 1999 Apr;20(2):101-35. doi: 10.1210/edrv.20.2.0361. Endocr Rev. 1999. PMID: 10204114 Review.
-
Dynamic duo: Kir6 and SUR in KATP channel structure and function.Channels (Austin). 2024 Dec;18(1):2327708. doi: 10.1080/19336950.2024.2327708. Epub 2024 Mar 15. Channels (Austin). 2024. PMID: 38489043 Free PMC article. Review.
Cited by
-
Functional analysis of six Kir6.2 (KCNJ11) mutations causing neonatal diabetes.Pflugers Arch. 2006 Dec;453(3):323-32. doi: 10.1007/s00424-006-0112-3. Epub 2006 Sep 22. Pflugers Arch. 2006. PMID: 17021801
-
ATP-sensitive K+ channels: regulation of bursting by the sulphonylurea receptor, PIP2 and regions of Kir6.2.J Physiol. 2006 Mar 1;571(Pt 2):303-17. doi: 10.1113/jphysiol.2005.100719. Epub 2005 Dec 22. J Physiol. 2006. PMID: 16373383 Free PMC article.
-
Molecular mechanism of pH sensing in KcsA potassium channels.Proc Natl Acad Sci U S A. 2008 May 13;105(19):6900-5. doi: 10.1073/pnas.0800873105. Epub 2008 Apr 28. Proc Natl Acad Sci U S A. 2008. PMID: 18443286 Free PMC article.
-
Single residue (K332A) substitution in Kir6.2 abolishes the stimulatory effect of long-chain acyl-CoA esters: indications for a long-chain acyl-CoA ester binding motif.Diabetologia. 2007 Aug;50(8):1670-7. doi: 10.1007/s00125-007-0697-x. Epub 2007 May 24. Diabetologia. 2007. PMID: 17522836
-
Determinants of trafficking, conduction, and disease within a K+ channel revealed through multiparametric deep mutational scanning.Elife. 2022 May 31;11:e76903. doi: 10.7554/eLife.76903. Elife. 2022. PMID: 35639599 Free PMC article.
References
-
- Aguilar-Bryan, L., and J. Bryan. 1999. Molecular biology of adenosine triphosphate-sensitive potassium channels. Endocr. Rev. 20:101–135. - PubMed
-
- Ashcroft, F.M., and F.M. Gribble. 1998. Correlating structure and function in ATP-sensitive K+ channels. Trends Neurosci. 21:288–294. - PubMed
-
- Baukrowitz, T., and B. Fakler. 2000. K(ATP) channels: linker between phospholipid metabolism and excitability. Biochem. Pharmacol. 60:735–740. - PubMed
-
- Baukrowitz, T., U. Schulte, D. Oliver, S. Herlitze, T. Krauter, S.J. Tucker, J.P. Ruppersberg, and B. Fakler. 1998. PIP2 and PIP as determinants for ATP inhibition of KATP channels. Science. 282:1141–1144. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

