External TEA block of shaker K+ channels is coupled to the movement of K+ ions within the selectivity filter
- PMID: 12885878
- PMCID: PMC2229542
- DOI: 10.1085/jgp.200308848
External TEA block of shaker K+ channels is coupled to the movement of K+ ions within the selectivity filter
Abstract
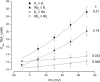
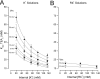
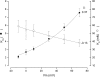
Recent molecular dynamic simulations and electrostatic calculations suggested that the external TEA binding site in K+ channels is outside the membrane electric field. However, it has been known for some time that external TEA block of Shaker K+ channels is voltage dependent. To reconcile these two results, we reexamined the voltage dependence of block of Shaker K+ channels by external TEA. We found that the voltage dependence of TEA block all but disappeared in solutions in which K+ ions were replaced by Rb+. These and other results with various concentrations of internal K+ and Rb+ ions suggest that the external TEA binding site is not within the membrane electric field and that the voltage dependence of TEA block in K+ solutions arises through a coupling with the movement of K+ ions through part of the membrane electric field. Our results suggest that external TEA block is coupled to two opposing voltage-dependent movements of K+ ions in the pore: (a) an inward shift of the average position of ions in the selectivity filter equivalent to a single ion moving approximately 37% into the pore from the external surface; and (b) a movement of internal K+ ions into a vestibule binding site located approximately 13% into the membrane electric field measured from the internal surface. The minimal voltage dependence of external TEA block in Rb+ solutions results from a minimal occupancy of the vestibule site by Rb+ ions and because the energy profile of the selectivity filter favors a more inward distribution of Rb+ occupancy.
Figures

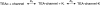
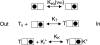
Similar articles
-
Two stable, conducting conformations of the selectivity filter in Shaker K+ channels.J Gen Physiol. 2005 Jun;125(6):619-29. doi: 10.1085/jgp.200509251. Epub 2005 May 16. J Gen Physiol. 2005. PMID: 15897293 Free PMC article.
-
Affinity and location of an internal K+ ion binding site in shaker K channels.J Gen Physiol. 2001 May;117(5):373-84. doi: 10.1085/jgp.117.5.373. J Gen Physiol. 2001. PMID: 11331347 Free PMC article.
-
Functional identification of ion binding sites at the internal end of the pore in Shaker K+ channels.J Physiol. 2003 May 15;549(Pt 1):107-20. doi: 10.1113/jphysiol.2002.038646. Epub 2003 Mar 28. J Physiol. 2003. PMID: 12665608 Free PMC article.
-
Synergistic inhibition of the maximum conductance of Kv1.5 channels by extracellular K+ reduction and acidification.Cell Biochem Biophys. 2005;43(2):231-42. doi: 10.1385/CBB:43:2:231. Cell Biochem Biophys. 2005. PMID: 16049348 Review.
-
Structure and function of potassium channels in plants: some inferences about the molecular origin of inward rectification in KAT1 channels (Review).Mol Membr Biol. 2003 Jan-Mar;20(1):19-25. doi: 10.1080/0968768021000057371. Mol Membr Biol. 2003. PMID: 12745922 Review.
Cited by
-
A cation-pi interaction between extracellular TEA and an aromatic residue in potassium channels.J Gen Physiol. 2006 Dec;128(6):649-57. doi: 10.1085/jgp.200609654. J Gen Physiol. 2006. PMID: 17130518 Free PMC article.
-
Ion selectivity and current saturation in inward-rectifier K+ channels.J Gen Physiol. 2012 Feb;139(2):145-57. doi: 10.1085/jgp.201110727. J Gen Physiol. 2012. PMID: 22291146 Free PMC article.
-
The external TEA binding site and C-type inactivation in voltage-gated potassium channels.Biophys J. 2004 Nov;87(5):3148-61. doi: 10.1529/biophysj.104.046664. Epub 2004 Aug 23. Biophys J. 2004. PMID: 15326027 Free PMC article.
-
Rapid intracellular TEA block of the KcsA potassium channel.Biophys J. 2005 Feb;88(2):1018-29. doi: 10.1529/biophysj.104.052043. Epub 2004 Nov 19. Biophys J. 2005. PMID: 15556975 Free PMC article.
-
Trans-channel interactions in batrachotoxin-modified skeletal muscle sodium channels: voltage-dependent block by cytoplasmic amines, and the influence of mu-conotoxin GIIIA derivatives and permeant ions.Biophys J. 2008 Nov 1;95(9):4277-88. doi: 10.1529/biophysj.108.138297. Epub 2008 Jul 25. Biophys J. 2008. PMID: 18658222 Free PMC article.

