Potent, persistent induction and modulation of cellular immune responses in rhesus macaques primed with Ad5hr-simian immunodeficiency virus (SIV) env/rev, gag, and/or nef vaccines and boosted with SIV gp120
- PMID: 12885879
- PMCID: PMC167211
- DOI: 10.1128/jvi.77.16.8607-8620.2003
Potent, persistent induction and modulation of cellular immune responses in rhesus macaques primed with Ad5hr-simian immunodeficiency virus (SIV) env/rev, gag, and/or nef vaccines and boosted with SIV gp120
Abstract
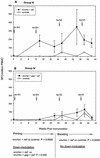
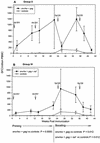
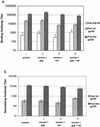
Immunity elicited by multicomponent vaccines delivered by replication-competent Ad5hr-simian immunodeficiency virus (SIV) recombinants was systematically investigated. Rhesus macaques were immunized mucosally at weeks 0 and 12 with Ad5hr-SIV(smH4) env/rev, with or without Ad5hr-SIV(mac239) gag or Ad5hr-SIV(mac239) nef, or with all three recombinants. The total Ad5hr dosage was comparably adjusted among all animals with empty Ad5hr-DeltaE3 vector. The macaques were boosted with SIV gp120 in monophosphoryl A-stable emulsion adjuvant at 24 and 36 weeks. Controls received Ad5hr-DeltaE3 vector or adjuvant only. By ELISPOT analysis, all four SIV gene products elicited potent cellular immune responses that persisted 42 weeks post-initial immunization. Unexpectedly, modulation of this cellular immune response was observed among macaques receiving one, two, or three Ad5hr-SIV recombinants. Env responses were significantly enhanced throughout the immunization period in macaques immunized with Ad5hr-SIV env/rev plus Ad5hr-SIV gag and tended to be higher in macaques that also received Ad5hr-SIV nef. Macaques primed with all three recombinants displayed significant down-modulation in numbers of gamma interferon (IFN-gamma)-secreting cells specific for SIV Nef, and the Env- and Gag-specific responses were also diminished. Modulation of antibody responses was not observed. Down-modulation was seen only during the period of Ad5hr-recombinant priming, not during subunit boosting, although SIV-specific IFN-gamma-secreting cells persisted. The effect was not attributable to Ad5hr replication differences among immunization groups. Vaccine delivery via replication-competent live vectors, which can persistently infect new cells and continuously present low-level antigen, may be advantageous in overcoming competition among complex immunogens for immune recognition. Effects of current multicomponent vaccines on individual immune responses should be evaluated with regard to future vaccine design.
Figures




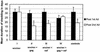
Similar articles
-
Boosting of SIV-specific immune responses in rhesus macaques by repeated administration of Ad5hr-SIVenv/rev and Ad5hr-SIVgag recombinants.Vaccine. 2003 Sep 8;21(25-26):4022-35. doi: 10.1016/s0264-410x(03)00266-4. Vaccine. 2003. PMID: 12922139
-
Improved protection of rhesus macaques against intrarectal simian immunodeficiency virus SIV(mac251) challenge by a replication-competent Ad5hr-SIVenv/rev and Ad5hr-SIVgag recombinant priming/gp120 boosting regimen.J Virol. 2003 Aug;77(15):8354-65. doi: 10.1128/jvi.77.15.8354-8365.2003. J Virol. 2003. PMID: 12857905 Free PMC article.
-
Potent, persistent cellular immune responses elicited by sequential immunization of rhesus macaques with Ad5 host range mutant recombinants encoding SIV Rev and SIV Nef.DNA Cell Biol. 2002 Sep;21(9):627-35. doi: 10.1089/104454902760330165. DNA Cell Biol. 2002. PMID: 12396605
-
SIV infected rhesus macaques: an AIDS model for immunoprevention and immunotherapy.Adv Exp Med Biol. 1989;251:279-93. doi: 10.1007/978-1-4757-2046-4_26. Adv Exp Med Biol. 1989. PMID: 2558527 Review.
-
Upregulation of Fas ligand by simian immunodeficiency virus - a nef-arious mechanism of immune evasion?J Exp Med. 1997 Jul 7;186(1):1-5. doi: 10.1084/jem.186.1.1. J Exp Med. 1997. PMID: 9207006 Free PMC article. Review. No abstract available.
Cited by
-
A replication-competent adenovirus-human immunodeficiency virus (Ad-HIV) tat and Ad-HIV env priming/Tat and envelope protein boosting regimen elicits enhanced protective efficacy against simian/human immunodeficiency virus SHIV89.6P challenge in rhesus macaques.J Virol. 2007 Apr;81(7):3414-27. doi: 10.1128/JVI.02453-06. Epub 2007 Jan 17. J Virol. 2007. PMID: 17229693 Free PMC article.
-
Replicating and non-replicating viral vectors for vaccine development.Curr Opin Biotechnol. 2007 Dec;18(6):546-56. doi: 10.1016/j.copbio.2007.10.010. Epub 2007 Dec 11. Curr Opin Biotechnol. 2007. PMID: 18063357 Free PMC article. Review.
-
Construction and immunogenicity of replication-competent adenovirus 5 host range mutant recombinants expressing HIV-1 gp160 of SF162 and TV1 strains.Vaccine. 2010 May 21;28(23):3963-71. doi: 10.1016/j.vaccine.2010.03.046. Epub 2010 Apr 9. Vaccine. 2010. PMID: 20382241 Free PMC article.
-
Assembly of pseudorabies virus genome-based transfer vehicle carrying major antigen sites of S gene of transmissible gastroenteritis virus: potential perspective for developing live vector vaccines.Biologicals. 2007 Mar;35(1):55-61. doi: 10.1016/j.biologicals.2006.02.001. Epub 2006 May 30. Biologicals. 2007. PMID: 16731004 Free PMC article.
-
Systemic neutralizing antibodies induced by long interval mucosally primed systemically boosted immunization correlate with protection from mucosal SHIV challenge.Virology. 2008 Dec 20;382(2):217-25. doi: 10.1016/j.virol.2008.09.016. Epub 2008 Oct 22. Virology. 2008. PMID: 18947849 Free PMC article.
References
-
- Amara, R. R., J. M. Smith, S. I. Staprans, D. C. Montefiori, F. Villinger, J. D. Altman, S. P. O'Neil, N. L. Kozyr, Y. Xu, L. S. Wyatt, P. L. Earl, J. G. Herndon, J. M. McNicholl, H. M. McClure, B. Moss, and H. L. Robinson. 2002. Critical role for Env as well as Gag-Pol in control of a simian-human immunodeficiency virus 89.6P challenge by a DNA prime/recombinant modified vaccinia virus Ankara vaccine. J. Virol. 76:6138-6146. - PMC - PubMed
-
- Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H.-L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. - PubMed
-
- Baba, T. W., V. Liska, R. Hofmann-Lehmann, J. Vlasak, W. Xu, S. Ayehunie, L. A. Cavacini, M. R. Posner, H. Katinger, G. Stiegler, B. J. Bernacky, T. A. Rizvi, R. Schmidt, L. R. Hill, M. E. Keeling, Y. Lu, J. E. Wright, T.-C. Chou, and R. M. Ruprecht. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat. Med. 6:200-206. - PubMed
-
- Baba, T. W., V. Liska, A. H. Khimani, N. B. Ray, P. J. Dailey, D. Penninck, R. Bronson, M. F. Greene, H. M. McClure, L. N. Martin, and R. M. Ruprecht. 1999. Live attenuated, multiply deleted simian immunodeficiency virus causes AIDS in infant and adult macaques. Nat. Med. 5:194-203. - PubMed
-
- Barouch, D. H., J. Kunstman, M. J. Kuroda, J. E. Schmitz, S. Santra, F. W. Peyeri, G. R. Krivulka, K. Beaudry, M. A. Lifton, D. A. Gorgone, D. C. Montefiori, M. G. Lewis, S. M. Wolinsky, and N. L. Letvin. 2002. Eventual AIDS vaccine failure in a rhesus monkey by viral escape from cytotoxic T lymphocytes. Nature 415:335-339. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

