Crimean-Congo hemorrhagic fever virus glycoprotein proteolytic processing by subtilase SKI-1
- PMID: 12885882
- PMCID: PMC167219
- DOI: 10.1128/jvi.77.16.8640-8649.2003
Crimean-Congo hemorrhagic fever virus glycoprotein proteolytic processing by subtilase SKI-1
Abstract
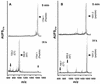
Crimean-Congo hemorrhagic fever (CCHF) virus is a tick-borne member of the genus Nairovirus, family Bunyaviridae. The mature virus glycoproteins, Gn and Gc (previously referred to as G2 and G1), are generated by proteolytic cleavage from precursor proteins. The amino termini of Gn and Gc are immediately preceded by tetrapeptides RRLL and RKPL, respectively, leading to the hypothesis that SKI-1 or related proteases may be involved (A. J. Sanchez, M. J. Vincent, and S. T. Nichol, J. Virol. 76:7263-7275, 2002). In vitro peptide cleavage data show that an RRLL peptide representing the Gn processing site is efficiently cleaved by SKI-1 protease, whereas an RKPL peptide representing the Gc processing site is cleaved at negligible levels. The efficient cleavage of RRLL peptide is consistent with the known recognition sequences of SKI-1, including the sequence determinants involved in the cleavage of the Lassa virus (family Arenaviridae) glycoprotein precursor. These in vitro findings were confirmed by expression of wild-type or mutant CCHF virus glycoproteins in CHO cells engineered to express functional or nonfunctional SKI-1. Gn processing was found to be dependent on functional SKI-1, whereas Gc processing was not. Gn processing occurred in the endoplasmic reticulum-cis Golgi compartments and was dependent on an R at the -4 position within the RRLL recognition motif, consistent with the known cleavage properties of SKI-1. Comparison of SKI-1 cleavage efficiency between peptides representing Lassa virus GP2 and CCHF virus Gn cleavage sites suggests that amino acids flanking the RRLL may modulate the efficiency. The apparent lack of SKI-1 cleavage at the CCHF virus Gc RKPL site indicates that related proteases, other than SKI-1, are likely to be involved in the processing at this site and identical or similar sites utilized in several New World arenaviruses.
Figures





Similar articles
-
Characterization of the glycoproteins of Crimean-Congo hemorrhagic fever virus.J Virol. 2002 Jul;76(14):7263-75. doi: 10.1128/jvi.76.14.7263-7275.2002. J Virol. 2002. PMID: 12072526 Free PMC article.
-
Crimean-congo hemorrhagic fever virus glycoprotein precursor is cleaved by Furin-like and SKI-1 proteases to generate a novel 38-kilodalton glycoprotein.J Virol. 2006 Jan;80(1):514-25. doi: 10.1128/JVI.80.1.514-525.2006. J Virol. 2006. PMID: 16352575 Free PMC article.
-
Crimean-Congo hemorrhagic fever virus glycoprotein processing by the endoprotease SKI-1/S1P is critical for virus infectivity.J Virol. 2007 Dec;81(23):13271-6. doi: 10.1128/JVI.01647-07. Epub 2007 Sep 26. J Virol. 2007. PMID: 17898072 Free PMC article.
-
Recent progress in molecular biology of Crimean-Congo hemorrhagic fever.Comp Immunol Microbiol Infect Dis. 2007 Sep;30(5-6):375-89. doi: 10.1016/j.cimid.2007.07.001. Epub 2007 Aug 10. Comp Immunol Microbiol Infect Dis. 2007. PMID: 17692916 Review.
-
Envelope glycoprotein of arenaviruses.Viruses. 2012 Oct 17;4(10):2162-81. doi: 10.3390/v4102162. Viruses. 2012. PMID: 23202458 Free PMC article. Review.
Cited by
-
Implication of proprotein convertases in the processing and spread of severe acute respiratory syndrome coronavirus.Biochem Biophys Res Commun. 2005 Jan 21;326(3):554-63. doi: 10.1016/j.bbrc.2004.11.063. Biochem Biophys Res Commun. 2005. PMID: 15596135 Free PMC article.
-
Novel approaches in anti-arenaviral drug development.Virology. 2011 Mar 15;411(2):163-9. doi: 10.1016/j.virol.2010.11.022. Epub 2010 Dec 22. Virology. 2011. PMID: 21183197 Free PMC article. Review.
-
Cellular localization and antigenic characterization of crimean-congo hemorrhagic fever virus glycoproteins.J Virol. 2005 May;79(10):6152-61. doi: 10.1128/JVI.79.10.6152-6161.2005. J Virol. 2005. PMID: 15858000 Free PMC article.
-
Analysis of the entry mechanism of Crimean-Congo hemorrhagic fever virus, using a vesicular stomatitis virus pseudotyping system.Arch Virol. 2016 Jun;161(6):1447-54. doi: 10.1007/s00705-016-2803-1. Epub 2016 Mar 3. Arch Virol. 2016. PMID: 26935918 Free PMC article.
-
Diverse Viruses Require the Calcium Transporter SPCA1 for Maturation and Spread.Cell Host Microbe. 2017 Oct 11;22(4):460-470.e5. doi: 10.1016/j.chom.2017.09.002. Cell Host Microbe. 2017. PMID: 29024641 Free PMC article.
References
-
- Basak, A., M. Zhong, J. S. Munzer, M. Chrétien, and N. G. Seidah. 2001. Implication of the proprotein convertases furin, PC5 and PC7 in the cleavage of surface glycoproteins of Hong Kong, Ebola and respiratory syncytial viruses - a comparative analysis using fluorogenic peptides. Biochem. J. 353:537-545. - PMC - PubMed
-
- Basak, A., M. Chrétien, and N. G. Seidah. 2002. A rapid fluorometric assay for the proteloytic activity of SKI-1/S1P based on the surface glycoprotein of the hemorrhagic fever Lassa virus. FEBS Lett. 514:333-339. - PubMed
-
- Brandt, W. E., E. L. Buescher, and F. M. Hetrick. 1967. Production and characterization of arbovirus antibody in mouse ascitic fluid. Am. J. Trop. Med. Hyg. 16:339-347. - PubMed
-
- Burns, J. W., and M. J. Buchmeier. 1993. Glycoproteins of the arenaviruses, p. 17-35. In M. S. Salvato (ed.), The Arenaviridae. Plenum Press, New York, N.Y.
-
- Decroly, E., M. Vandenbranden, J. M. Ruysschaert, J. Cogniaux, G. S. Jacob, S. C. Howard, G. Marshall, A. Kompelli, A. Basak, F. Jean, C. Lazure, S. Benjannet, M. Chrétien, R. Day, and N. G. Seidah. 1994. The convertases furin and PC1 can both cleave the human immunodeficiency virus (HIV)-1 envelope glycoprotein gp160 into gp120 (HIV-I SU) and gp41 (HIV-I TM). J. Biol. Chem. 269:12240-12247. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

