Nonstructural proteins NS1 and NS2 of bovine respiratory syncytial virus block activation of interferon regulatory factor 3
- PMID: 12885884
- PMCID: PMC167228
- DOI: 10.1128/jvi.77.16.8661-8668.2003
Nonstructural proteins NS1 and NS2 of bovine respiratory syncytial virus block activation of interferon regulatory factor 3
Abstract
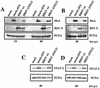
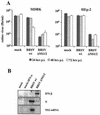
We have previously shown that the nonstructural (NS) proteins NS1 and NS2 of bovine respiratory syncytial virus (BRSV) mediate resistance to the alpha/beta interferon (IFN)-mediated antiviral response. Here, we show that they, in addition, are able to prevent the induction of beta IFN (IFN-beta) after virus infection or double-stranded RNA stimulation. In BRSV-infected MDBK cells upregulation of IFN-stimulated genes (ISGs) such as MxA did not occur, although IFN signaling via JAK/STAT was found intact. In contrast, infection with recombinant BRSVs lacking either or both NS genes resulted in efficient upregulation of ISGs. Biological IFN activity and IFN-beta were detected only in supernatants of cells infected with the NS deletion mutants but not with wild-type (wt) BRSV. Subsequent analyses of IFN-beta promoter activity showed that infection of cells with the double deletion mutant BRSV DeltaNS1/2, but not with BRSV wt, resulted in a significant increase in IFN-beta gene promoter activity. Induction of the IFN-beta promoter depends on the activation of three distinct transcription factors, NF-kappaB, ATF-2/c-Jun, and IFN regulatory factor 3 (IRF-3). Whereas NF-kappaB and ATF-2/c-Jun activities were readily detectable and comparable in both wt BRSV- and BRSV DeltaNS1/2-infected cells, phosphorylation and transcriptional activity of IRF-3, however, were observed only after BRSV DeltaNS1/2 infection. NS protein-mediated inhibition of IRF-3 activation and IFN induction should have considerable impact on the pathogenesis and immunogenicity of BRSV.
Figures




References
-
- Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675-680. - PubMed
-
- Alexopoulou, L., A. C. Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413:732-738. - PubMed
-
- Andrejeva, J., D. F. Young, S. Goodbourn, and R. E. Randall. 2002. Degradation of STAT1 and STAT2 by the V proteins of simian virus 5 and human parainfluenza virus type 2, respectively: consequences for virus replication in the presence of alpha/beta and gamma interferons. J. Virol. 76:2159-2167. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

