Newcastle disease virus V protein is associated with viral pathogenesis and functions as an alpha interferon antagonist
- PMID: 12885886
- PMCID: PMC167241
- DOI: 10.1128/jvi.77.16.8676-8685.2003
Newcastle disease virus V protein is associated with viral pathogenesis and functions as an alpha interferon antagonist
Abstract
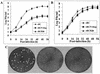
Newcastle disease virus (NDV) edits its P gene by inserting one or two G residues at the conserved editing site (UUUUUCCC, genome sense) and transcribes the P mRNA (unedited), the V mRNA (with a +1 frameshift), and the W mRNA (with a +2 frameshift). All three proteins are amino coterminal but vary at their carboxyl terminus in length and amino acid composition. Little is known about the role of the V and W proteins in NDV replication and pathogenesis. We have constructed and recovered two recombinant viruses in which the expression of the V or both the V and W proteins has been abolished. Compared to the parental virus, the mutant viruses showed impaired growth in cell cultures, except in Vero cells. However, transient expression of the carboxyl-terminal portion of the V protein enhanced the growth of the mutant viruses. In embryonated chicken eggs, the parental virus grew to high titers in embryos of different gestational ages, whereas the mutant viruses showed an age-dependent phenomenon, growing to lower titer in more-developed embryos. An interferon (IFN) sensitivity assay showed that the parental virus was more resistant to the antiviral effect of IFN than the mutant viruses. Moreover, infection with the parental virus resulted in STAT1 protein degradation, but not with the mutant viruses. These findings indicate that the V protein of NDV possesses the ability to inhibit alpha IFN and that the IFN inhibitory function lies in the carboxyl-terminal domain. Pathogenicity studies showed that the V protein of NDV significantly contributes to the virus virulence.
Figures





Similar articles
-
A recombinant newcastle disease virus with low-level V protein expression is immunogenic and lacks pathogenicity for chicken embryos.J Virol. 2001 Jan;75(1):420-8. doi: 10.1128/JVI.75.1.420-428.2001. J Virol. 2001. PMID: 11119610 Free PMC article.
-
Newcastle disease virus V protein is a determinant of host range restriction.J Virol. 2003 Sep;77(17):9522-32. doi: 10.1128/jvi.77.17.9522-9532.2003. J Virol. 2003. PMID: 12915566 Free PMC article.
-
Identification of a mutation in editing of defective Newcastle disease virus recombinants that modulates P-gene mRNA editing and restores virus replication and pathogenicity in chicken embryos.J Virol. 2003 Sep;77(17):9259-65. doi: 10.1128/jvi.77.17.9259-9265.2003. J Virol. 2003. PMID: 12915541 Free PMC article.
-
Newcastle disease virus (NDV)-based assay demonstrates interferon-antagonist activity for the NDV V protein and the Nipah virus V, W, and C proteins.J Virol. 2003 Jan;77(2):1501-11. doi: 10.1128/jvi.77.2.1501-1511.2003. J Virol. 2003. PMID: 12502864 Free PMC article.
-
Overexpression of Newcastle disease virus (NDV) V protein enhances NDV production kinetics in chicken embryo fibroblasts.Appl Microbiol Biotechnol. 2010 Feb;85(5):1509-20. doi: 10.1007/s00253-009-2189-z. Appl Microbiol Biotechnol. 2010. PMID: 19730851
Cited by
-
Viral Inhibition of the IFN-Induced JAK/STAT Signalling Pathway: Development of Live Attenuated Vaccines by Mutation of Viral-Encoded IFN-Antagonists.Vaccines (Basel). 2016 Jun 29;4(3):23. doi: 10.3390/vaccines4030023. Vaccines (Basel). 2016. PMID: 27367734 Free PMC article. Review.
-
Evasion of Host Antiviral Innate Immunity by Paramyxovirus Accessory Proteins.Front Microbiol. 2022 Jan 31;12:790191. doi: 10.3389/fmicb.2021.790191. eCollection 2021. Front Microbiol. 2022. PMID: 35173691 Free PMC article. Review.
-
Applications of Gene Editing in Chickens: A New Era Is on the Horizon.Front Genet. 2018 Oct 9;9:456. doi: 10.3389/fgene.2018.00456. eCollection 2018. Front Genet. 2018. PMID: 30356667 Free PMC article. Review.
-
The cytoplasmic location of chicken mx is not the determining factor for its lack of antiviral activity.PLoS One. 2010 Aug 16;5(8):e12151. doi: 10.1371/journal.pone.0012151. PLoS One. 2010. PMID: 20808435 Free PMC article.
-
A Novel Chimeric Oncolytic Virus Vector for Improved Safety and Efficacy as a Platform for the Treatment of Hepatocellular Carcinoma.J Virol. 2018 Nov 12;92(23):e01386-18. doi: 10.1128/JVI.01386-18. Print 2018 Dec 1. J Virol. 2018. PMID: 30232179 Free PMC article.
References
-
- Alexander, D. J. 1989. Newcastle disease, p. 114-120. In H. G. Purchase, L. H. Arp, C. H. Domermuth, and J. E. Pearson (ed.), A laboratory manual for the isolation and identification of avian pathogens, 3rd ed. American Association for Avian Pathologists, Inc., Kennett Square, Pa.
-
- Alexander, D. J. 1997. Newcastle disease and other avian Paramyxoviridae infections, p. 541-569. In B. W. Calnek (ed.), Diseases of poultry, 10th ed. Iowa State University Press, Ames.
-
- Andrejeva, J., D. F. Young, S. Goodbourn, and R. E. Randall. 2002. Degradation of STAT1 and STAT2 by the V protein of simian virus 5 and human parainfluenza virus type 2, respectively: consequence for virus replication in the presence of alpha/beta and gamma interferons. J. Virol. 76:2159-2167. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

