Plasmid chemokines and colony-stimulating factors enhance the immunogenicity of DNA priming-viral vector boosting human immunodeficiency virus type 1 vaccines
- PMID: 12885892
- PMCID: PMC167238
- DOI: 10.1128/jvi.77.16.8729-8735.2003
Plasmid chemokines and colony-stimulating factors enhance the immunogenicity of DNA priming-viral vector boosting human immunodeficiency virus type 1 vaccines
Abstract
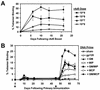
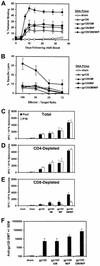
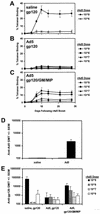
Heterologous "prime-boost" regimens that involve priming with plasmid DNA vaccines and boosting with recombinant viral vectors have been shown to elicit potent virus-specific cytotoxic T-lymphocyte responses. Increasing evidence, however, suggests that the utility of recombinant viral vectors in human populations will be significantly limited by preexisting antivector immunity. Here we demonstrate that the coadministration of plasmid chemokines and colony-stimulating factors with plasmid DNA vaccines markedly increases the immunogenicity of DNA prime-recombinant adenovirus serotype 5 (rAd5) boost and DNA prime-recombinant vaccinia virus (rVac) boost vaccine regimens in BALB/c mice. In mice with preexisting anti-Ad5 immunity, priming with the DNA vaccine alone followed by rAd5 boosting elicited only marginal immune responses. In contrast, cytokine-augmented DNA vaccine priming followed by rAd5 vector boosting was able to generate potent immune responses in mice with preexisting anti-Ad5 immunity. These data demonstrate that plasmid cytokines can markedly improve the immunogenicity of DNA prime-viral vector boost vaccine strategies and can partially compensate for antivector immunity.
Figures


References
-
- Altman, J. D., P. A. H. Moss, P. J. R. Goulder, D. H. Barouch, M. G. McHeyzer-Williams, J. I. Bell, A. J. McMichael, and M. M. Davis. 1996. Phenotypic analysis of antigen-specific T lymphocytes. Science 274:94-96. - PubMed
-
- Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H.-K. Ma, B. D. Grimm, M. L. Hulsey, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of clinical AIDS in rhesus monkeys by a multiprotein DNA/MVA vaccine. Science 292:69-74. - PubMed
-
- Barouch, D. H., S. Santra, T. D. Steenbeke, X. X. Zheng, H. C. Perry, M.-E. Davies, D. C. Freed, A. Craiu, T. B. Strom, J. W. Shiver, and N. L. Letvin. 1998. Augmentation and suppression of immune responses to an HIV-1 DNA vaccine by plasmid cytokine/Ig administration. J. Immunol. 161:1875-1882. - PubMed
-
- Barouch, D. H., S. Santra, K. Tenner-Racz, P. Racz, M. J. Kuroda, J. E. Schmitz, S. S. Jackson, M. A. Lifton, D. C. Freed, H. C. Perry, M. E. Davies, J. W. Shiver, and N. L. Letvin. 2002. Potent CD4+ T cell responses elicited by a bicistronic HIV-1 DNA vaccine expressing gp120 and GM-CSF. J. Immunol. 168:562-568. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

