Identification of the E9/E2C cDNA and functional characterization of the gene product reveal a new repressor of transcription and replication in cottontail rabbit papillomavirus
- PMID: 12885893
- PMCID: PMC167252
- DOI: 10.1128/jvi.77.16.8736-8744.2003
Identification of the E9/E2C cDNA and functional characterization of the gene product reveal a new repressor of transcription and replication in cottontail rabbit papillomavirus
Abstract
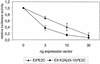
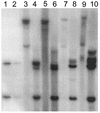
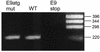
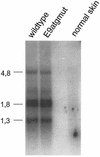
Cottontail rabbit papillomavirus (CRPV) genomes mutated in the trans-activation domain of the E2 protein, which stimulates both viral DNA replication and transcription, are severely impaired in their ability to induce tumors in New Zealand White rabbits. A number of papillomaviruses encode, in addition to full-length E2, a shortened E2 protein or an E2 protein fused to a short stretch of amino acids derived from the small E8 open reading frame that counteract the activities of E2. We identified and cloned the novel cDNA E9/E2C of CRPV from papillomas of New Zealand White and cottontail rabbits and characterized the functions of the encoded gene product. E9/E2C was shown to be a bona fide repressor of minimal viral promoters, with the E9 domain being essential for this activity, and to repress E1/E2-dependent replication of a CRPV origin construct. In addition, E9/E2C counteracted the transactivation effect of the full-length E2 on minimal promoters containing several E2 binding sites. To investigate the role of E9/E2C in tumorigenesis, we constructed two CRPV genomes mutated in E9/E2C. One, designated CRPV-E9atgmut-pLAII, contained a mutation in the unique start codon in the E9 open reading frame, and the second E9/E2C mutant was constructed by the introduction of a stop codon close to the splice donor site at nucleotide 3714 that additionally prevented the correct splicing of the transcript. When we infected New Zealand White rabbits with these constructs, we surprisingly noted no differences in tumor induction efficiency, viral genome copy number, and viral transcription in comparison to wild-type CRPV.
Figures





Similar articles
-
A transactivator function of cottontail rabbit papillomavirus e2 is essential for tumor induction in rabbits.J Virol. 2002 Nov;76(22):11209-15. doi: 10.1128/jvi.76.22.11209-11215.2002. J Virol. 2002. PMID: 12388680 Free PMC article.
-
A recombinant cottontail rabbit papillomavirus genome for ectopic expression of genes in cells infected with virus in vivo.J Virol Methods. 2013 Jan;187(1):110-3. doi: 10.1016/j.jviromet.2012.09.008. Epub 2012 Sep 24. J Virol Methods. 2013. PMID: 23018059
-
Variation in the nucleotide sequence of cottontail rabbit papillomavirus a and b subtypes affects wart regression and malignant transformation and level of viral replication in domestic rabbits.J Virol. 2000 Nov;74(22):10766-77. doi: 10.1128/jvi.74.22.10766-10777.2000. J Virol. 2000. PMID: 11044121 Free PMC article.
-
Cottontail rabbit papillomavirus (CRPV) model system to test antiviral and immunotherapeutic strategies.Antivir Chem Chemother. 2005;16(6):355-62. doi: 10.1177/095632020501600602. Antivir Chem Chemother. 2005. PMID: 16331841 Review.
-
Cottontail Rabbit Papillomavirus (CRPV) Related Animal Models for Head and Neck Cancer Research: A Comprehensive Review of the Literature.Viruses. 2024 Oct 31;16(11):1722. doi: 10.3390/v16111722. Viruses. 2024. PMID: 39599834 Free PMC article. Review.
Cited by
-
Inhibition of transcription and DNA replication by the papillomavirus E8-E2C protein is mediated by interaction with corepressor molecules.J Virol. 2008 Jun;82(11):5127-36. doi: 10.1128/JVI.02647-07. Epub 2008 Mar 19. J Virol. 2008. PMID: 18353941 Free PMC article.
-
A novel recombinant papillomavirus genome enabling in vivo RNA interference reveals that YB-1, which interacts with the viral regulatory protein E2, is required for CRPV-induced tumor formation in vivo.Am J Cancer Res. 2014 May 26;4(3):222-33. eCollection 2014. Am J Cancer Res. 2014. PMID: 24959377 Free PMC article.
-
Papillomavirus-Associated Tumor Formation Critically Depends on c-Fos Expression Induced by Viral Protein E2 and Bromodomain Protein Brd4.PLoS Pathog. 2016 Jan 4;12(1):e1005366. doi: 10.1371/journal.ppat.1005366. eCollection 2016 Jan. PLoS Pathog. 2016. PMID: 26727473 Free PMC article.
-
The viral E4 protein is required for the completion of the cottontail rabbit papillomavirus productive cycle in vivo.J Virol. 2004 Feb;78(4):2142-51. doi: 10.1128/jvi.78.4.2142-2151.2004. J Virol. 2004. PMID: 14747580 Free PMC article.
-
Modeling HPV-Associated Disease and Cancer Using the Cottontail Rabbit Papillomavirus.Viruses. 2022 Sep 4;14(9):1964. doi: 10.3390/v14091964. Viruses. 2022. PMID: 36146770 Free PMC article. Review.
References
-
- Berkhout, R. J., L. M. Tieben, H. L. Smits, J. N. Bavinck, B. J. Vermeer, and J. ter Schegget. 1995. Nested PCR approach for detection and typing of epidermodysplasia verruciformis-associated human papillomavirus types in cutaneous cancers from renal transplant recipients. J. Clin. Microbiol. 33:690-695. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

