Feline immunodeficiency virus ORF-Ais required for virus particle formation and virus infectivity
- PMID: 12885901
- PMCID: PMC167212
- DOI: 10.1128/jvi.77.16.8819-8830.2003
Feline immunodeficiency virus ORF-Ais required for virus particle formation and virus infectivity
Abstract
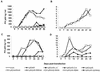
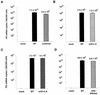
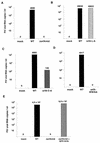
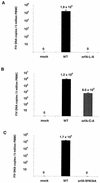
The orf-A (orf-2) gene of feline immunodeficiency virus (FIV) is a small open reading frame predicted to encode a 77-amino-acid protein that contains putative domains similar to those of the ungulate lentiviral Tat protein. Orf-A is reported to be critical for efficient viral replication in vitro and in vivo. A series of FIV-pPPR-derived proviruses with in-frame deletions and point mutations within orf-A were constructed and tested for replication in feline lymphoid cells. Orf-A mutant proviruses were also tested for viral gene and protein expression, viral particle formation, and virion infectivity. Deletions within orf-A severely restricted FIV replication in feline peripheral blood mononuclear cells (PBMC) and interleukin-2-dependent T-cell lines. In addition, substitutions of alanines for leucines in the putative leucine-rich domain, for cysteines in the putative cysteine-rich domain, and for a tryptophan at position 43 in Orf-A restricted the replication of FIV mutants. Deletions and point mutations in orf-A imposed a small effect or no effect on FIV long-terminal-repeat-driven viral gene expression and had no effect on viral protein expression. However, release of cell-free, virion-associated viral RNA in supernatants from cells transfected with orf-A mutant proviruses was severely restricted but was rescued by cotransfection with a wild-type Orf-A expression vector. In addition, virions derived from orf-A mutant proviruses expressed reduced infectivity for feline PBMC. Our findings suggest that Orf-A functions involve multiple steps of the FIV life cycle including both virion formation and infectivity. Furthermore, these observations suggest that Orf-A represents an FIV-encoded analog more similar to the accessory gene vpr, vpu, or nef than to the regulatory gene tat encoded by the primate lentiviruses.
Figures


Similar articles
-
Feline immunodeficiency virus Orf-A localizes to the nucleus and induces cell cycle arrest.Virology. 2004 Aug 1;325(2):167-74. doi: 10.1016/j.virol.2004.05.007. Virology. 2004. PMID: 15246256
-
Identification of a feline immunodeficiency virus gene which is essential for cell-free virus infectivity.J Virol. 1992 Oct;66(10):6181-5. doi: 10.1128/JVI.66.10.6181-6185.1992. J Virol. 1992. PMID: 1382146 Free PMC article.
-
The genome of feline immunodeficiency virus.Arch Virol. 1994;134(3-4):221-34. doi: 10.1007/BF01310563. Arch Virol. 1994. PMID: 8129613 Review.
-
The feline immunodeficiency virus ORF-A gene facilitates efficient viral replication in established T-cell lines and peripheral blood lymphocytes.J Virol. 1993 Oct;67(10):5889-95. doi: 10.1128/JVI.67.10.5889-5895.1993. J Virol. 1993. PMID: 7690413 Free PMC article.
-
In vivo functions of the auxiliary genes and regulatory elements of feline immunodeficiency virus.Vet Microbiol. 1998 Feb 28;60(2-4):141-53. doi: 10.1016/s0378-1135(98)00157-6. Vet Microbiol. 1998. PMID: 9646446 Review.
Cited by
-
Feline immunodeficiency virus (FIV) as a model for study of lentivirus infections: parallels with HIV.Curr HIV Res. 2010 Jan;8(1):73-80. doi: 10.2174/157016210790416389. Curr HIV Res. 2010. PMID: 20210782 Free PMC article. Review.
-
Transcriptional regulation of latent feline immunodeficiency virus in peripheral CD4+ T-lymphocytes.Viruses. 2012 May;4(5):878-88. doi: 10.3390/v4050878. Epub 2012 May 23. Viruses. 2012. PMID: 22754653 Free PMC article.
-
Feline immunodeficiency virus latency.Retrovirology. 2013 Jul 6;10:69. doi: 10.1186/1742-4690-10-69. Retrovirology. 2013. PMID: 23829177 Free PMC article. Review.
-
Molecular mechanisms of FIV infection.Vet Immunol Immunopathol. 2008 May 15;123(1-2):3-13. doi: 10.1016/j.vetimm.2008.01.007. Epub 2008 Jan 19. Vet Immunol Immunopathol. 2008. PMID: 18289701 Free PMC article. Review.
-
Characterization of antiviral T cell responses during primary and secondary challenge of laboratory cats with feline infectious peritonitis virus (FIPV).BMC Vet Res. 2019 May 22;15(1):165. doi: 10.1186/s12917-019-1909-6. BMC Vet Res. 2019. PMID: 31118053 Free PMC article.
References
-
- Barlough, J. E., C. D. Ackley, J. W. George, N. Levy, R. Acevedo, P. F. Moore, B. A. Rideout, M. D. Cooper, and N. C. Pedersen. 1991. Acquired immune dysfunction in cats with experimentally induced feline immunodeficiency virus infection: comparison of short-term and long-term infections. J. Acquir. Immune Defic. Syndr. 4:219-227. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

