Investigation of structural and functional motifs within the vaccinia virus A14 phosphoprotein, an essential component of the virion membrane
- PMID: 12885904
- PMCID: PMC167248
- DOI: 10.1128/jvi.77.16.8857-8871.2003
Investigation of structural and functional motifs within the vaccinia virus A14 phosphoprotein, an essential component of the virion membrane
Abstract
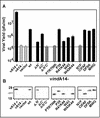
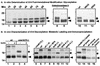
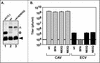
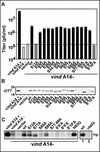
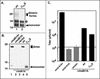
We have previously reported the construction and characterization of an inducible recombinant virus in which expression of the vaccinia virus membrane protein A14 is experimentally regulated using the tetracycline operator-repressor system. Repression of A14, which results in a 1,000-fold reduction in viral yield, leads to an early block in viral morphogenesis characterized by the accumulation of large virosomes, empty "crescents" that fail to contact these virosomes, and, most strikingly, large numbers of aberrant 25-nm vesicles. Here we report the establishment of a transient-complementation system for the structure-function analysis of A14. We have constructed numerous mutant alleles of A14 designed to identify and test the importance of key structural and sequence motifs within A14, including sites of posttranslational modification, such as glycosylation, phosphorylation, and dimerization. From these studies we have determined that robust complementation ability requires an intact N terminus and two regions flanking the first membrane-spanning domain of A14. We show that A14 is modified by N-linked glycosylation both in vitro and in vivo. However, only a minority of A14 molecules are glycosylated in vivo and these are not encapsidated. In this report we also identify the sole phosphorylated serine residue of A14 as lying within the NHS(85) motif that undergoes glycosylation. Additionally, we show that the Cys(71) residue is required for intermolecular disulfide bond formation and describe the properties of a virus expressing an allele of A14 that cannot form disulfide-linked dimers.
Figures


References
-
- Bogan, A. A., and K. S. Thorn. 1998. Anatomy of hot spots in protein interfaces. J. Mol. Biol. 280:1-9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

