Residues in the apical domain of the feline and canine transferrin receptors control host-specific binding and cell infection of canine and feline parvoviruses
- PMID: 12885908
- PMCID: PMC167234
- DOI: 10.1128/jvi.77.16.8915-8923.2003
Residues in the apical domain of the feline and canine transferrin receptors control host-specific binding and cell infection of canine and feline parvoviruses
Abstract
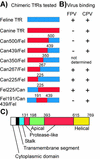
Canine parvovirus (CPV) and feline panleukopenia virus (FPV) capsids bind to the transferrin receptors (TfRs) of their hosts and use these receptors to infect cells. The binding is partially host specific, as FPV binds only to the feline TfR, while CPV binds to both the canine and feline TfRs. The host-specific binding is controlled by a combination of residues within a raised region of the capsid. To define the TfR structures that interact with the virus, we altered the apical domain of the feline or canine TfR or prepared chimeras of these receptors and tested the altered receptors for binding to FPV or CPV capsids. Most changes in the apical domain of the feline TfR did not affect binding, but replacing Leu221 with Ser or Asp prevented receptor binding to either FPV or CPV capsids, while replacing Leu221 with Lys resulted in a receptor that bound only to CPV but not to FPV. Analysis of recombinants of the feline and canine TfRs showed that sequences controlling CPV-specific binding were within the apical domain and that more than one difference between these receptors determined the CPV-specific binding of the canine TfR. Single changes within the canine TfR which removed a single amino acid insertion or which eliminated a glycosylation site gave that receptor the expanded ability to bind to FPV and CPV. In some cases, binding of capsids to mutant receptors did not result in infection, suggesting a structural role for the receptor in cell infection by the viruses.
Figures



Similar articles
-
Purified feline and canine transferrin receptors reveal complex interactions with the capsids of canine and feline parvoviruses that correspond to their host ranges.J Virol. 2006 Sep;80(17):8482-92. doi: 10.1128/JVI.00683-06. J Virol. 2006. PMID: 16912298 Free PMC article.
-
Binding site on the transferrin receptor for the parvovirus capsid and effects of altered affinity on cell uptake and infection.J Virol. 2010 May;84(10):4969-78. doi: 10.1128/JVI.02623-09. Epub 2010 Mar 3. J Virol. 2010. PMID: 20200243 Free PMC article.
-
Combinations of two capsid regions controlling canine host range determine canine transferrin receptor binding by canine and feline parvoviruses.J Virol. 2003 Sep;77(18):10099-105. doi: 10.1128/jvi.77.18.10099-10105.2003. J Virol. 2003. PMID: 12941920 Free PMC article.
-
[Evolution and host variation of the canine parvovirus: molecular basis for the development of a new virus].Berl Munch Tierarztl Wochenschr. 2004 Mar-Apr;117(3-4):130-5. Berl Munch Tierarztl Wochenschr. 2004. PMID: 15046459 Review. German.
-
Pathogenesis of feline panleukopenia virus and canine parvovirus.Baillieres Clin Haematol. 1995 Mar;8(1):57-71. doi: 10.1016/s0950-3536(05)80232-x. Baillieres Clin Haematol. 1995. PMID: 7663051 Free PMC article. Review.
Cited by
-
Diagnostic Challenges in Canine Parvovirus 2c in Vaccine Failure Cases.Viruses. 2020 Sep 3;12(9):980. doi: 10.3390/v12090980. Viruses. 2020. PMID: 32899378 Free PMC article.
-
Limited Intrahost Diversity and Background Evolution Accompany 40 Years of Canine Parvovirus Host Adaptation and Spread.J Virol. 2019 Dec 12;94(1):e01162-19. doi: 10.1128/JVI.01162-19. Print 2019 Dec 12. J Virol. 2019. PMID: 31619551 Free PMC article.
-
Host receptors: the key to establishing cells with broad viral tropism for vaccine production.Crit Rev Microbiol. 2020 Mar;46(2):147-168. doi: 10.1080/1040841X.2020.1735992. Epub 2020 Mar 23. Crit Rev Microbiol. 2020. PMID: 32202955 Free PMC article. Review.
-
Identification of a Feline Panleukopenia Virus from Captive Giant Pandas (Ailuropoda melanoleuca) and Its Phylogenetic Analysis.Transbound Emerg Dis. 2023 May 19;2023:7721487. doi: 10.1155/2023/7721487. eCollection 2023. Transbound Emerg Dis. 2023. PMID: 40303714 Free PMC article.
-
Limited transferrin receptor clustering allows rapid diffusion of canine parvovirus into clathrin endocytic structures.J Virol. 2012 May;86(9):5330-40. doi: 10.1128/JVI.07194-11. Epub 2012 Feb 22. J Virol. 2012. PMID: 22357278 Free PMC article.
References
-
- Agbandje, M., R. McKenna, M. G. Rossmann, M. L. Strassheim, and C. R. Parrish. 1993. Structure determination of feline panleukopenia virus empty particles. Proteins 16:155-171. - PubMed
-
- Bates, G. W., and M. R. Schlabach. 1973. The reaction of ferric salts with transferrin. J. Biol. Chem. 248:3228-3232. - PubMed
-
- Bates, G. W., and J. Wernicke. 1971. The kinetics and mechanism of iron(3) exchange between chelates and transferrin. IV. The reaction of transferrin with iron(3) nitrilotriacetate. J. Biol. Chem. 246:3679-3685. - PubMed
-
- Bennett, M. J., J. A. Lebron, and P. J. Bjorkman. 2000. Crystal structure of the hereditary haemochromatosis protein HFE complexed with transferrin receptor. Nature 403:46-53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

