Local delivery of CpG oligodeoxynucleotides induces rapid changes in the genital mucosa and inhibits replication, but not entry, of herpes simplex virus type 2
- PMID: 12885911
- PMCID: PMC167233
- DOI: 10.1128/jvi.77.16.8948-8956.2003
Local delivery of CpG oligodeoxynucleotides induces rapid changes in the genital mucosa and inhibits replication, but not entry, of herpes simplex virus type 2
Abstract
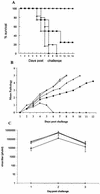
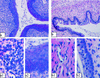
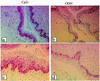
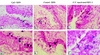
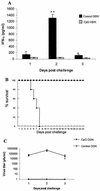
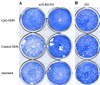
Mucosal surfaces are the entry sites for the vast majority of infectious pathogens and provide the first line of defense against infection. In addition to the epithelial barrier, the innate immune system plays a key role in recognizing and rapidly responding to invading pathogens via innate receptors, such as Toll-like receptors (TLR). Bacterial CpG DNA, a potent activator of innate immunity, is recognized by TLR9. Here, we confirm that local mucosal, but not systemic, delivery of CpG oligodeoxynucleotides (ODN) to the genital tract protects mice from a subsequent lethal vaginal herpes simplex virus type 2 (HSV-2) challenge. Since these effects were so local in action, we examined the genital mucosa. Local delivery of CpG ODN induced rapid proliferation and thickening of the genital epithelium and caused significant recruitment of inflammatory cells to the submucosa. Local CpG ODN treatment also resulted in inhibition of HSV-2 replication but had no effect on HSV-2 entry into the genital mucosa. CpG ODN-induced protection against HSV-2 was not associated with early increases in gamma interferon (IFN-gamma) secretion in the genital tract, and CpG ODN-treated IFN-gamma(-/-) mice were protected from subsequent challenge with a lethal dose of HSV-2. Treatment of human HEK-293 cells transfected with murine TLR9 showed that the antiviral activity of CpG ODN was mediated through TLR9. These studies suggest that local induction of mucosal innate immunity can provide protection against sexually transmitted infections, such as HSV-2 or possibly human immunodeficiency virus, at the mucosal surfaces.
Figures


References
-
- Adler, H., J. L. Beland, N. C. Del-Pan, L. Kobzik, R. A. Sobel, and I. J. Rimm. 1999. In the absence of T cells, natural killer cells protect from mortality due to HSV-1 encephalitis. J. Neuroimmunol. 93:208-213. - PubMed
-
- Ashkar, A. A., and K. L. Rosenthal. 2002. Toll-like receptor 9, CpG DNA and innate immunity. Curr. Mol. Med. 2:545-556. - PubMed
-
- Chang, M. A., J. W. Horner, B. R. Conklin, R. A. DePinho, D. Bok, and D. J. Zack. 2000. Tetracycline-inducible system for photoreceptor-specific gene expression. Investig. Ophthalmol. Vis. Sci. 41:4281-4287. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

