A high-molecular-weight complex of membrane proteins BAP29/BAP31 is involved in the retention of membrane-bound IgD in the endoplasmic reticulum
- PMID: 12886015
- PMCID: PMC187866
- DOI: 10.1073/pnas.1633363100
A high-molecular-weight complex of membrane proteins BAP29/BAP31 is involved in the retention of membrane-bound IgD in the endoplasmic reticulum
Abstract
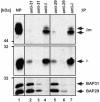
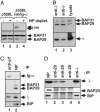
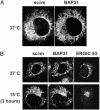
B cell antigen receptors (BCRs) are multimeric transmembrane protein complexes comprising membrane-bound immunoglobulins (mIgs) and Ig-alpha/Ig-beta heterodimers. In most cases, transport of mIgs from the endoplasmic reticulum (ER) to the cell surface requires assembly with the Ig-alpha/Ig-beta subunits. In addition to Ig-alpha/Ig-beta, mIg molecules also bind two ER-resident membrane proteins, BAP29 and BAP31, and the chaperone heavy chain binding protein (BiP). In this article, we show that neither Ig-alpha/Ig-beta nor BAP29/BAP31 nor BiP bind simultaneously to the same mIgD molecule. Blue native PAGE revealed that only a minor fraction of intracellular mIgD is associated with high-molecular-weight BAP29/BAP31 complexes. BAP-binding to mIgs was found to correlate with ER retention of chimeric mIgD molecules. On high-level expression in Drosophila melanogaster S2 cells, mIgD molecules were detected on the cell surface in the absence of Ig-alpha/Ig-beta. This aberrant transport was prevented by coexpression of BAP29 and BAP31. Thus, BAP complexes contribute to ER retention of mIg complexes that are not bound to Ig-alpha/Ig-beta. Furthermore, the mechanism of ER retention of both BAP31 and mIgD is not through retrieval from a post-ER compartment, but true ER retention. In conclusion, BAP29 and BAP31 might be the long sought after retention proteins and/or chaperones that act on transmembrane regions of various proteins.
Figures



References
-
- Reth, M. (1992) Annu. Rev. Immunol. 10, 97–121. - PubMed
-
- Reth, M., Wienands, J. & Schamel, W. W. A. (2000) Immunol. Rev. 176, 10–18. - PubMed
-
- Reth, M. (1989) Nature 338, 383–384. - PubMed
-
- Wienands, J. (1999) The B Cell Antigen Receptor: Formation of Signaling Complexes and the Function of Adaptor Proteins (Springer, Berlin). - PubMed
-
- Benschop, R. J. & Cambier, J. C. (1999) Curr. Opin. Immunol. 11, 143–151. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

