Structural diversification and neo-functionalization during floral MADS-box gene evolution by C-terminal frameshift mutations
- PMID: 12888499
- PMCID: PMC169922
- DOI: 10.1093/nar/gkg642
Structural diversification and neo-functionalization during floral MADS-box gene evolution by C-terminal frameshift mutations
Abstract
Frameshift mutations generally result in loss-of-function changes since they drastically alter the protein sequence downstream of the frameshift site, besides creating premature stop codons. Here we present data suggesting that frameshift mutations in the C-terminal domain of specific ancestral MADS-box genes may have contributed to the structural and functional divergence of the MADS-box gene family. We have identified putative frameshift mutations in the conserved C-terminal motifs of the B-function DEF/AP3 subfamily, the A-function SQUA/AP1 subfamily and the E-function AGL2 subfamily, which are all involved in the specification of organ identity during flower development. The newly evolved C-terminal motifs are highly conserved, suggesting a de novo generation of functionality. Interestingly, since the new C-terminal motifs in the A- and B-function subfamilies are only found in higher eudicotyledonous flowering plants, the emergence of these two C-terminal changes coincides with the origin of a highly standardized floral structure. We speculate that the frameshift mutations described here are examples of co-evolution of the different components of a single transcription factor complex. 3' terminal frameshift mutations might provide an important but so far unrecognized mechanism to generate novel functional C-terminal motifs instrumental to the functional diversification of transcription factor families.
Figures



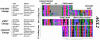

References
-
- Theissen G., Becker,A., Di Rosa,A., Kanno,A., Kim,J.T., Munster,T., Winter,K.U. and Saedler,H. (2000) A short history of MADS-box genes in plants. Plant Mol. Biol., 42, 115–149. - PubMed
-
- Theissen G., Kim,J.T. and Saedler,H. (1996) Classification and phylogeny of the MADS-box multigene family suggest defined roles of MADS-box gene subfamilies in the morphological evolution of eukaryotes. J. Mol. Evol., 43, 484–516. - PubMed
-
- Theissen G. (2001) Development of floral organ identity: stories from the MADS house. Curr. Opin. Plant Biol., 4, 75–85. - PubMed
-
- Pelaz S., Ditta,G.S., Baumann,E., Wisman,E. and Yanofsky,M.F. (2000) B and C floral organ identity functions require SEPALLATA MADS-box genes. Nature, 405, 200–203. - PubMed
-
- Liljegren S.J., Ditta,G.S., Eshed,Y., Savidge,B., Bowman,J.L. and Yanofsky,M.F. (2000) SHATTERPROOF MADS-box genes control seed dispersal in Arabidopsis. Nature, 404, 766–770. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

