Complex effects of NMDA receptor antagonist APV in the basolateral amygdala on acquisition of two-way avoidance reaction and long-term fear memory
- PMID: 12888548
- PMCID: PMC202320
- DOI: 10.1101/lm.58803
Complex effects of NMDA receptor antagonist APV in the basolateral amygdala on acquisition of two-way avoidance reaction and long-term fear memory
Abstract
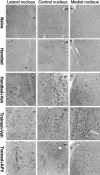
Although much has been learned about the role of the amygdala in Pavlovian fear conditioning, relatively little is known about an involvement of this structure in more complex aversive learning, such as acquisition of an active avoidance reaction. In the present study, rats with a pretraining injection of the N-methyl-D-aspartate (NMDA) receptor antagonist, 2-amino-5-phosphonopentanoic acid (APV), into the basolateral amygdala (BLA) were found to be impaired in two-way active avoidance learning. During multitrial training in a shuttle box, the APV-injected rats were not different from the controls in sensitivity to shock or in acquisition of freezing to contextual cues. However, APV injection led to impaired retention of contextual fear when tested 48 h later, along with an attenuation of c-Fos expression in the amygdala. These results are consistent with the role of NMDA receptors of the BLA in long-term memory of fear, previously documented in Pavlovian conditioning paradigms. The APV-induced impairment in the active avoidance learning coincided with deficits in directionality of the escape reaction and in attention to conditioned stimuli. These data indicate that normal functioning of NMDA receptors in the basolateral amygdala is required during acquisition of adaptive instrumental responses in a shuttle box but is not necessary for acquisition of short-term contextual fear in this situation.
Figures



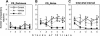




Similar articles
-
N-methyl-D-aspartate receptors in the basolateral amygdala are required for both acquisition and expression of conditional fear in rats.Behav Neurosci. 1996 Dec;110(6):1365-74. doi: 10.1037//0735-7044.110.6.1365. Behav Neurosci. 1996. PMID: 8986338
-
Pretraining NMDA receptor blockade in the basolateral complex, but not the central nucleus, of the amygdala prevents savings of conditional fear.Behav Neurosci. 2003 Aug;117(4):738-50. doi: 10.1037/0735-7044.117.4.738. Behav Neurosci. 2003. PMID: 12931959
-
NMDA receptor antagonism in the basolateral but not central amygdala blocks the extinction of Pavlovian fear conditioning in rats.Eur J Neurosci. 2010 May;31(9):1664-70. doi: 10.1111/j.1460-9568.2010.07223.x. Eur J Neurosci. 2010. PMID: 20525079 Free PMC article.
-
Synaptic transmission and plasticity in the amygdala. An emerging physiology of fear conditioning circuits.Mol Neurobiol. 1996 Aug;13(1):1-22. doi: 10.1007/BF02740749. Mol Neurobiol. 1996. PMID: 8892333 Review.
-
The Neural Foundations of Reaction and Action in Aversive Motivation.Curr Top Behav Neurosci. 2016;27:171-95. doi: 10.1007/7854_2015_401. Curr Top Behav Neurosci. 2016. PMID: 26643998 Review.
Cited by
-
An animal model of emotional blunting in schizophrenia.PLoS One. 2007 Dec 26;2(12):e1360. doi: 10.1371/journal.pone.0001360. PLoS One. 2007. PMID: 18159243 Free PMC article.
-
Contribution of emotional and motivational neurocircuitry to cue-signaled active avoidance learning.Front Behav Neurosci. 2014 Oct 27;8:372. doi: 10.3389/fnbeh.2014.00372. eCollection 2014. Front Behav Neurosci. 2014. PMID: 25386127 Free PMC article. Review. No abstract available.
-
Natural activation of caspase-3 is required for the development of operant behavior in postnatal ontogenesis.Neurosci Behav Physiol. 2009 Jan;39(1):65-72. doi: 10.1007/s11055-008-9097-z. Epub 2008 Dec 17. Neurosci Behav Physiol. 2009. PMID: 19089628
-
Involvement of cyclin-dependent kinase-like 2 in cognitive function required for contextual and spatial learning in mice.Front Behav Neurosci. 2010 Apr 19;4:17. doi: 10.3389/fnbeh.2010.00017. eCollection 2010. Front Behav Neurosci. 2010. PMID: 20428496 Free PMC article.
-
Stress-Induced Enhanced Long-Term Potentiation and Reduced Threshold for N-Methyl-D-Aspartate Receptor- and β-Adrenergic Receptor-Mediated Synaptic Plasticity in Rodent Ventral Subiculum.Front Mol Neurosci. 2021 Apr 22;14:658465. doi: 10.3389/fnmol.2021.658465. eCollection 2021. Front Mol Neurosci. 2021. PMID: 33967694 Free PMC article.
References
-
- Aronin, N., Chase, K., Sagar, S.M., Sharp, F.R., and DiFiglia, M. 1991. N-Methyl-D-aspartate receptor activation in the neostriatum increases c-fos and fos-related antigens selectively in medium-sized neurons. Neuroscience 44: 409–420. - PubMed
-
- Bignami, G., Alleva, A., Amorico, L., De Acetis, L., and Giardini, V. 1985. Bidirectional avoidance by mice as a function of CS, US, and apparatus variables. Animal Learn. Behav. 13: 439–450.
-
- Bolles, R.C. 1979. Learning theory, 2nd ed. Holt, Rinehart and Winston, New York.
-
- Bures, J., Buresova, O., and Huston, J.P. 1983. Techniques and basic experiments for the study of brain and behavior, 2nd ed. Elsevier, Amsterdam–NY.
-
- Cahill, L. and McGaugh, J.L. 1998. Mechanisms of emotional arousal and lasting declarative memory. Trends Neurosci. 21: 294–299. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
