Caspase cleavage of tau: linking amyloid and neurofibrillary tangles in Alzheimer's disease
- PMID: 12888622
- PMCID: PMC187753
- DOI: 10.1073/pnas.1630428100
Caspase cleavage of tau: linking amyloid and neurofibrillary tangles in Alzheimer's disease
Abstract
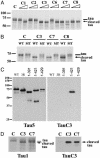
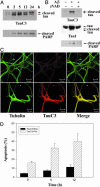
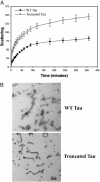
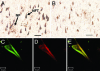
The principal pathological features of Alzheimer's disease (AD) are extracellular amyloid plaques and intracellular neurofibrillary tangles, the latter composed of the microtubule-binding protein tau assembled into paired helical and straight filaments. Recent studies suggest that these pathological entities may be functionally linked, although the mechanisms by which amyloid deposition promotes pathological tau filament assembly are poorly understood. Here, we report that tau is proteolyzed by multiple caspases at a highly conserved aspartate residue (Asp421) in its C terminus in vitro and in neurons treated with amyloid-beta (Abeta) (1-42) peptide. Tau is rapidly cleaved at Asp421 in Abeta-treated neurons (within 2 h), and its proteolysis appears to precede the nuclear events of apoptosis. We also demonstrate that caspase cleavage of tau generates a truncated protein that lacks its C-terminal 20 amino acids and assembles more rapidly and more extensively into tau filaments in vitro than wild-type tau. Using a monoclonal antibody that specifically recognizes tau truncated at Asp421, we show that tau is proteolytically cleaved at this site in the fibrillar pathologies of AD brain. Taken together, our results suggest a novel mechanism linking amyloid deposition and neurofibrillary tangles in AD: Abeta peptides promote pathological tau filament assembly in neurons by triggering caspase cleavage of tau and generating a proteolytic product with enhanced polymerization kinetics.
Figures
References
-
- Yuan, J. & Yankner, B. A. (2000) Nature 407, 802–809. - PubMed
-
- Trojanowski, J. Q., Schmidt, M. L., Shin, R. W., Bramblett, G. T., Rao, D. & Lee, V. M. (1993) Brain Pathol. 3, 45–54. - PubMed
-
- Hardy, J. & Selkoe, D. J. (2002) Science 297, 353–356. - PubMed
-
- Hutton, M., Lendon, C. L., Rizzu, P., Baker, M., Froelich, S., Houlden, H., Pickering-Brown, S., Chakraverty, S., Isaacs, A., Grover, A., et al. (1998) Nature 393, 702–705. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

