West Nile virus in farmed alligators
- PMID: 12890319
- PMCID: PMC3023431
- DOI: 10.3201/eid0907.030085
West Nile virus in farmed alligators
Abstract
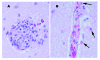
Seven alligators were submitted to the Tifton Veterinary Diagnostic and Investigational Laboratory for necropsy during two epizootics in the fall of 2001 and 2002. The alligators were raised in temperature-controlled buildings and fed a diet of horsemeat supplemented with vitamins and minerals. Histologic findings in the juvenile alligators were multiorgan necrosis, heterophilic granulomas, and heterophilic perivasculitis and were most indicative of septicemia or bacteremia. Histologic findings in a hatchling alligator were random foci of necrosis in multiple organs and mononuclear perivascular encephalitis, indicative of a viral cause. West Nile virus was isolated from submissions in 2002. Reverse transcription-polymerase chain reaction (RT-PCR) results on all submitted case samples were positive for West Nile virus for one of four cases associated with the 2001 epizootic and three of three cases associated with the 2002 epizootic. RT-PCR analysis was positive for West Nile virus in the horsemeat collected during the 2002 outbreak but negative in the horsemeat collected after the outbreak.
Figures

References
-
- Doi R, Oya A, Shirasaka A, Yabe S, Sasa M. Studies on Japanese encephalitis virus infection of reptiles. II. Role of lizards on hibernation of Japanese encephalitis virus. Jpn J Exp Med. 1983;53:125–34. - PubMed
-
- McLean RG, Ubico SR, Bourne D, Komar N. West Nile virus in livestock and wildlife. Curr Top Microbiol Immunol. 2002;267:271–308. - PubMed
-
- Nir Y, Lasowski Y, Avivi A, Cgoldwasser R. Survey for antibodies to arboviruses in the serum of various animals in Israel during 1965–1966. Am J Trop Med Hyg. 1969;18:416–22. - PubMed
-
- Thomas LA, Patzer ER, Cory JC, Coe JE. Antibody development in garter snakes (Thamnophis spp.) experimentally infected with western equine encephalitis virus. Am J Trop Med Hyg. 1980;29:112–7. - PubMed
-
- Oya A, Doi R, Shirasaka A, Yabe S, Sasa M. Studies on Japanese encephalitis virus infection of reptiles. I. Experimental infection of snakes and lizards. Jpn J Exp Med. 1983;53:117–23. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
