Malondialdehyde inhibits cardiac contractile function in ventricular myocytes via a p38 mitogen-activated protein kinase-dependent mechanism
- PMID: 12890710
- PMCID: PMC1573967
- DOI: 10.1038/sj.bjp.0705384
Malondialdehyde inhibits cardiac contractile function in ventricular myocytes via a p38 mitogen-activated protein kinase-dependent mechanism
Abstract
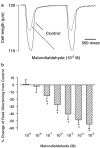
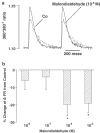
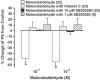
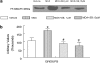
(1) Increased oxidative stress plays a significant role in the etiology of cardiovascular disease. Lipid peroxidation, initiated in the presence of hydroxy radicals resulting in the production of malondialdehyde, directly produces oxidative stress. This study was designed to examine the direct impact of malondialdehyde on ventricular contractile function at the single cardiac myocyte level. Ventricular myocytes from adult rat hearts were stimulated to contract at 0.5 Hz, and mechanical and intracellular Ca(2+) properties were evaluated using an IonOptix Myocam system. Contractile properties analyzed included peak shortening amplitude (PS), time-to-PS (TPS), time-to-90% relengthening (TR(90)), maximal velocity of shortening/relengthening (+/-dLdt), and Ca(2+)-induced intracellular Ca(2+) fluorescence release (CICR) and intracellular Ca(2+) decay (tau). p38 mitogen-activated protein (MAP) kinase phosphorylation was assessed with Western blot. (2) Our results indicated that malondialdehyde directly depressed PS, +/-dLdt and CICR in a concentration-dependent manner and shortened TPS without affecting TR(90) and tau. Interestingly, the malondialdehyde-induced cardiac mechanical effect was abolished by both the p38 MAP kinase inhibitor SB203580 (1 and 10 micro M) and the antioxidant vitamin C (100 micro M). Western blot analysis confirmed direct phosphorylation of p38 MAP kinase by malondialdehyde. (3) These findings revealed a novel role of malondialdehyde and p38 MAP kinase in lipid peroxidation and oxidative stress-associated cardiac dysfunction.
Figures
Similar articles
-
Streptozotocin directly impairs cardiac contractile function in isolated ventricular myocytes via a p38 map kinase-dependent oxidative stress mechanism.Biochem Biophys Res Commun. 2004 Jun 11;318(4):1066-71. doi: 10.1016/j.bbrc.2004.04.138. Biochem Biophys Res Commun. 2004. PMID: 15147982
-
Inhibition of cardiac myocyte contraction by 4-hydroxy-trans-2-nonenal.Cardiovasc Toxicol. 2004;4(1):21-8. doi: 10.1385/ct:4:1:21. Cardiovasc Toxicol. 2004. PMID: 15034202
-
Insulin resistance induces hyperleptinemia, cardiac contractile dysfunction but not cardiac leptin resistance in ventricular myocytes.Int J Obes Relat Metab Disord. 2003 Oct;27(10):1196-203. doi: 10.1038/sj.ijo.0802389. Int J Obes Relat Metab Disord. 2003. PMID: 14513067
-
Doxorubicin induces cardiomyocyte dysfunction via a p38 MAP kinase-dependent oxidative stress mechanism.Cancer Detect Prev. 2005;29(3):294-9. doi: 10.1016/j.cdp.2004.07.008. Epub 2004 Nov 23. Cancer Detect Prev. 2005. PMID: 15936597
-
The oxygen radical generator pyrogallol impairs cardiomyocyte contractile function via a superoxide and p38 MAP kinase-dependent pathway: protection by anisodamine and tetramethylpyrazine.Cardiovasc Toxicol. 2004;4(4):375-84. doi: 10.1385/ct:4:4:375. Cardiovasc Toxicol. 2004. PMID: 15531780
Cited by
-
Detrimental Effects of Lipid Peroxidation in Type 2 Diabetes: Exploring the Neutralizing Influence of Antioxidants.Antioxidants (Basel). 2022 Oct 20;11(10):2071. doi: 10.3390/antiox11102071. Antioxidants (Basel). 2022. PMID: 36290794 Free PMC article. Review.
-
DNA-Dependent Protein Kinase Catalytic Subunit Prevents Ferroptosis in Retinal Pigment Epithelial Cells.Invest Ophthalmol Vis Sci. 2025 Jan 2;66(1):50. doi: 10.1167/iovs.66.1.50. Invest Ophthalmol Vis Sci. 2025. PMID: 39841110 Free PMC article.
-
Cinnamomum burmannii (Nees & T. Nees) Blume and Eleutherine palmifolia (L.) Merr. extract combination ameliorate lipid profile and heart oxidative stress in hyperlipidemic mice.Vet World. 2020 Jul;13(7):1404-1409. doi: 10.14202/vetworld.2020.1404-1409. Epub 2020 Jul 22. Vet World. 2020. PMID: 32848317 Free PMC article.
-
Zinc nanoparticles ameliorated obesity-induced cardiovascular disease: role of metabolic syndrome and iron overload.Sci Rep. 2023 Sep 25;13(1):16010. doi: 10.1038/s41598-023-42550-y. Sci Rep. 2023. PMID: 37749096 Free PMC article.
-
Nox2 Upregulation and p38α MAPK Activation in Right Ventricular Hypertrophy of Rats Exposed to Long-Term Chronic Intermittent Hypobaric Hypoxia.Int J Mol Sci. 2020 Nov 13;21(22):8576. doi: 10.3390/ijms21228576. Int J Mol Sci. 2020. PMID: 33202984 Free PMC article.
References
-
- BERLETT B.S., STADTMAN E.R. Protein oxidation in aging, disease, and oxidative stress. J. Biol. Chem. 1997;272:20313–20316. - PubMed
-
- BRAUGHLER J.M., DUNCAN L.A., CHASE R.L. The involvement of iron in lipid peroxidation. Importance of ferric to ferrous ratios in initiation. J. Biol. Chem. 1986;261:10282–10289. - PubMed
-
- BUFFON A., SANTINI S.A., RAMAZZOTTI V., RIGATTIERI S., LIUZZO G., BIASUCCI L.M., CREA F., GIARDINA B., MASERI A. Large, sustained cardiac lipid peroxidation and reduced antioxidant capacity in the coronary circulation after brief episodes of myocardial ischemia. J. Am. Coll. Cardiol. 2000;35:633–639. - PubMed
-
- BURTON G.W., FOSTER D.O., PERLY B., SLATER T.F., SMITH I.C.P., INGOLD K.U. Biological antioxidants. Philos Trans. R. Soc. London B. 1985;311:565–578. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

