Rapid critical period induction by tonic inhibition in visual cortex
- PMID: 12890762
- PMCID: PMC6740711
- DOI: 10.1523/JNEUROSCI.23-17-06695.2003
Rapid critical period induction by tonic inhibition in visual cortex
Abstract
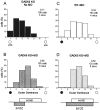
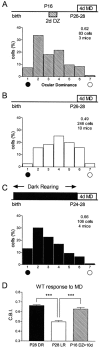
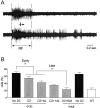
Mice lacking a synaptic isoform of glutamic acid decarboxylase (GAD65) do not exhibit ocular dominance plasticity unless an appropriate level of GABAergic transmission is restored by direct infusion of benzodiazepines into the brain. To better understand how intracortical inhibition triggers experience-dependent changes, we dissected the precise timing requirement for GABA function in the monocular deprivation (MD) paradigm. Diazepam (DZ) or vehicle solution was infused daily before and/or during 4 d of MD in GAD65 knock-out mice. Extracellular single-unit recordings from the binocular zone of visual cortex were performed at the end of deprivation. We found that a minimum treatment of 2 d near the beginning of MD was sufficient to fully activate plasticity but did not need to overlap the deprivation per se. Extended delay after DZ infusion eventually led to loss of plasticity accompanied by improved intrinsic inhibitory circuit function. Two day DZ treatment just after eye opening similarly closed the critical period prematurely in wild-type mice. Raising wild-type mice in complete darkness from birth delayed the peak sensitivity to MD as in other mammals. Interestingly, 2 d DZ infusion in the dark also closed the critical period, whereas equally brief light exposure during dark-rearing had no such effect. Thus, enhanced tonic signaling through GABA(A) receptors rapidly creates a milieu for plasticity within neocortex capable of triggering a critical period for ocular dominance independent of visual experience itself.
Figures




Similar articles
-
Regional Specificity of GABAergic Regulation of Cross-Modal Plasticity in Mouse Visual Cortex after Unilateral Enucleation.J Neurosci. 2015 Aug 12;35(32):11174-89. doi: 10.1523/JNEUROSCI.3808-14.2015. J Neurosci. 2015. PMID: 26269628 Free PMC article.
-
Cross-modal Restoration of Juvenile-like Ocular Dominance Plasticity after Increasing GABAergic Inhibition.Neuroscience. 2018 Nov 21;393:1-11. doi: 10.1016/j.neuroscience.2018.09.040. Epub 2018 Oct 6. Neuroscience. 2018. PMID: 30300702
-
Permissive proteolytic activity for visual cortical plasticity.Proc Natl Acad Sci U S A. 2002 May 28;99(11):7717-21. doi: 10.1073/pnas.102088899. Proc Natl Acad Sci U S A. 2002. PMID: 12032349 Free PMC article.
-
Plasticity in the visual system: role of neurotrophins and electrical activity.Arch Ital Biol. 2002 Oct;140(4):341-6. Arch Ital Biol. 2002. PMID: 12228987 Review.
-
Critical-period plasticity in the visual cortex.Annu Rev Neurosci. 2012;35:309-30. doi: 10.1146/annurev-neuro-061010-113813. Epub 2012 Mar 29. Annu Rev Neurosci. 2012. PMID: 22462544 Review.
Cited by
-
Regional Specificity of GABAergic Regulation of Cross-Modal Plasticity in Mouse Visual Cortex after Unilateral Enucleation.J Neurosci. 2015 Aug 12;35(32):11174-89. doi: 10.1523/JNEUROSCI.3808-14.2015. J Neurosci. 2015. PMID: 26269628 Free PMC article.
-
Early pharmacological treatment of autism: a rationale for developmental treatment.Biol Psychiatry. 2007 Feb 15;61(4):521-37. doi: 10.1016/j.biopsych.2006.09.021. Biol Psychiatry. 2007. PMID: 17276749 Free PMC article. Review.
-
Developmental changes in GABAergic mechanisms in human visual cortex across the lifespan.Front Cell Neurosci. 2010 Jun 10;4:16. doi: 10.3389/fncel.2010.00016. eCollection 2010. Front Cell Neurosci. 2010. PMID: 20592950 Free PMC article.
-
Parvalbumin-Positive Interneurons Regulate Cortical Sensory Plasticity in Adulthood and Development Through Shared Mechanisms.Front Neural Circuits. 2022 May 6;16:886629. doi: 10.3389/fncir.2022.886629. eCollection 2022. Front Neural Circuits. 2022. PMID: 35601529 Free PMC article. Review.
-
Visual deprivation decreases somatic GAD65 puncta number on layer 2/3 pyramidal neurons in mouse visual cortex.Neural Plast. 2009;2009:415135. doi: 10.1155/2009/415135. Epub 2009 May 25. Neural Plast. 2009. PMID: 19503840 Free PMC article.
References
-
- Arellano JI, DeFelipe J, Munoz A ( 2002) PSA-NCAM immunoreactivity in chandelier cell axon terminals of the human temporal cortex. Cereb Cortex 12: 617-624. - PubMed
-
- Asada H, Kawamura Y, Maruyama K, Kume H, Ding R, Ji FY, Kanbara N, Kuzume H, Sanbo M, Yagi T, Obata K ( 1996) Mice lacking the 65 kDa isoform of glutamic acid decarboxylase (GAD65) maintain normal levels of GAD67 and GABA in their brains but are susceptible to seizures. Biochem Biophys Res Commun 229: 891-895. - PubMed
-
- Beaver CJ, Ji Q-H, Daw NW ( 2001) Layer differences in the effect of monocular vision in light and dark reared kittens. Vis Neurosci 18: 811-820. - PubMed
-
- Benevento LA, Bakkum BW, Port JD, Cohen RS ( 1992) The effects of dark-rearing on the electrophysiology of the rat visual cortex. Brain Res 572: 198-207. - PubMed
-
- Benevento LA, Bakkum BW, Cohen RS ( 1995) gamma-Aminobutyric acid and somatostatin immunoreactivity in the visual cortex of normal and dark-reared rats. Brain Res 689: 172-182. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
