The relay of high-frequency sensory signals in the Whisker-to-barreloid pathway
- PMID: 12890771
- PMCID: PMC6740730
- DOI: 10.1523/JNEUROSCI.23-17-06778.2003
The relay of high-frequency sensory signals in the Whisker-to-barreloid pathway
Abstract
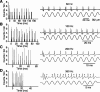
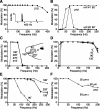
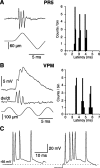
The present study investigated the operational features of whisker-evoked EPSPs in barreloid cells and the ability of the whisker-to-barreloid pathway to relay high rates of whisker deflection in lightly anesthetized rats. Results show that lemniscal EPSPs are single-fiber events with fast rise times (<500 microsec) that strongly depress at short inter-EPSP intervals. They occur at short latencies (3.84 +/- 0.96 msec) with little jitters (<300 microsec) after electrical stimulation of the whisker follicle. Waveform analysis indicates that one to three lemniscal axons converge on individual barreloid cells to produce EPSPs of similar rise times but different amplitudes. When challenged by high rates of whisker deflection, cells in the whisker-to-barreloid pathway demonstrate a remarkable frequency-following ability. Primary vibrissa afferents could follow in a phase-locked manner trains of sinusoidal deflections at up to 1 kHz. Although trigeminothalamic cells could still faithfully follow deflection rates of 200-300 Hz, the actual frequency-following ability of individual cells depends on the amplitude, velocity, and direction of displacements. The discharges of trigeminothalamic cells induce corresponding phase-locked EPSPs in barreloid cells, which trigger burst discharges at stimulus onset. During the following cycles of the stimulus train, few action potentials ensue because of the strong synaptic depression at lemniscal synapses. It is concluded that the whisker-to-barreloid pathway can relay vibratory inputs with a high degree of temporal precision, but that the relay of this information to the cerebral cortex requires the action of modulators, and possibly phase-locked discharges among an ensemble of relay cells.
Figures







References
-
- Ahissar E, Sosnik R, Haidarliu S ( 2000) Transformation from temporal to rate coding in a somatosensory thalamocortical pathway. Nature 406: 302-306. - PubMed
-
- Alonso JM, Usrey WM, Reid RC ( 1996) Precisely correlated firing in cells of the lateral geniculate nucleus. Nature 383: 815-819. - PubMed
-
- Bae YC, Ihn HJ, Park MJ, Otterson OP, Moritani M, Yoshida A, Yoshio S ( 2000) Identification of signal substances in synapses made between primary afferents and their associated axon terminals in the rat trigeminal sensory nuclei. J Comp Neurol 418: 299-309. - PubMed
-
- Bloomfield SA, Sherman MS ( 1988) Postsynaptic potentials recorded in neurons of the cat's lateral geniculate nucleus following electrical stimulation of the optic chiasm. J Neurophysiol 60: 1924-1945. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
