Coherent oscillations in neuronal activity of the supplementary motor area during a visuomotor task
- PMID: 12890774
- PMCID: PMC6740733
- DOI: 10.1523/JNEUROSCI.23-17-06798.2003
Coherent oscillations in neuronal activity of the supplementary motor area during a visuomotor task
Abstract
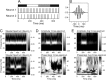
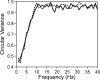
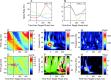
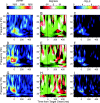
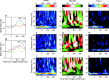
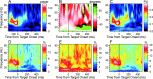
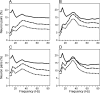
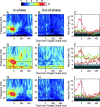
Neural activity recorded in behaving animals is nonstationary, making it difficult to determine factors influencing its temporal patterns. In the present study, rhesus monkeys were trained to produce a series of visually guided hand movements according to the changes in target locations, and multichannel single-neuron activity was recorded from the caudal supplementary motor area. Coherent oscillations in neural activity were analyzed using the wavelet cross-spectrum, and its statistical significance was evaluated using various methods based on surrogate spike trains and trial shuffling. A population-averaged wavelet cross-spectrum displayed a strong tendency for oscillatory activity in the gamma frequency range (30 approximately 50 Hz) to synchronize immediately before and after the onset of movement target. The duration of synchronized oscillations in the gamma frequency range increased when the onset of the next target was delayed. In addition, analysis of individual neuron pairs revealed that many neuron pairs also displayed coherent oscillations in the beta frequency range (15-30 Hz). Coherent beta frequency oscillations were less likely to be synchronized than gamma frequency oscillations, consistent with the fact that coherent beta frequency oscillations were not clearly seen in the population-averaged cross-spectrum. For a given neuron pair, the time course and phase of coherent oscillations were often similar across different movements. These results are consistent with the proposal that synchronized oscillations in the gamma frequency range might be related to the anticipation of behaviorally relevant events and the contextual control of cortical information flow.
Figures
References
-
- Aertsen AMHJ, Gerstein GL, Habib MK, Palm G ( 1989) Dynamics of neuronal firing correlation: modulation of “effective connectivity.” J Neurophysiol 61: 900-917. - PubMed
-
- Andrew C, Pfurtscheller G ( 1996) Event-related coherence as a tool for studying dynamic interaction of brain regions. Electroencephalogr Clin Neurophysiol 98: 144-148. - PubMed
-
- Aoki F, Fetz EE, Shupe L, Lettich E, Ojemann GA ( 1999) Increased gamma-range activity in human sensorimotor cortex during performance of visuomotor task. Clin Neurophysiol 110: 524-537. - PubMed
-
- Baker SN, Lemon RN ( 2000) Precise spatiotemporal repeating patterns in monkey primary and supplementary motor areas occur at chance levels. J Neurophysiol 84: 1770-1780. - PubMed
-
- Baker SN, Spinks R, Jackson A, Lemon RN ( 2001) Synchronization in monkey motor cortex during a precision grip task. I. Task-dependent modulation in single-unit synchrony. J Neurophysiol 85: 869-885. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
