Enhanced expression of a specific hyperpolarization-activated cyclic nucleotide-gated cation channel (HCN) in surviving dentate gyrus granule cells of human and experimental epileptic hippocampus
- PMID: 12890777
- PMCID: PMC3100807
- DOI: 10.1523/JNEUROSCI.23-17-06826.2003
Enhanced expression of a specific hyperpolarization-activated cyclic nucleotide-gated cation channel (HCN) in surviving dentate gyrus granule cells of human and experimental epileptic hippocampus
Abstract
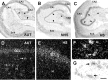
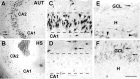
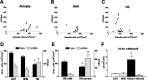
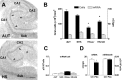
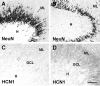
Changes in the expression of ion channels, contributing to altered neuronal excitability, are emerging as possible mechanisms in the development of certain human epilepsies. In previous immature rodent studies of experimental prolonged febrile seizures, isoform-specific changes in the expression of hyperpolarization-activated cyclic nucleotide-gated cation channels (HCNs) correlated with long-lasting hippocampal hyperexcitability and enhanced seizure susceptibility. Prolonged early-life seizures commonly precede human temporal lobe epilepsy (TLE), suggesting that transcriptional dysregulation of HCNs might contribute to the epileptogenic process. Therefore, we determined whether HCN isoform expression was modified in hippocampi of individuals with TLE. HCN1 and HCN2 expression were measured using in situ hybridization and immunocytochemistry in hippocampi from three groups: TLE with hippocampal sclerosis (HS; n = 17), epileptic hippocampi without HS, or non-HS (NHS; n = 10), and autopsy material (n = 10). The results obtained in chronic human epilepsy were validated by examining hippocampi from the pilocarpine model of chronic TLE. In autopsy and most NHS hippocampi, HCN1 mRNA expression was substantial in pyramidal cell layers and lower in dentate gyrus granule cells (GCs). In contrast, HCN1 mRNA expression over the GC layer and in individual GCs from epileptic hippocampus was markedly increased once GC neuronal density was reduced by >50%. HCN1 mRNA changes were accompanied by enhanced immunoreactivity in the GC dendritic fields and more modest changes in HCN2 mRNA expression. Furthermore, similar robust and isoform-selective augmentation of HCN1 mRNA expression was evident also in the pilocarpine animal model of TLE. These findings indicate that the expression of HCN isoforms is dynamically regulated in human as well as in experimental hippocampal epilepsy. After experimental febrile seizures (i.e., early in the epileptogenic process), the preserved and augmented inhibition onto principal cells may lead to reduced HCN1 expression. In contrast, in chronic epileptic HS hippocampus studied here, the profound loss of interneuronal and principal cell populations and consequent reduced inhibition, coupled with increased dendritic excitation of surviving GCs, might provoke a "compensatory" enhancement of HCN1 mRNA and protein expression.
Figures

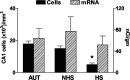

References
-
- Abercrombie M ( 1946) Estimation of nuclear population from microtome sections. Anat Rec 94: 239-247. - PubMed
-
- Armstrong DD ( 1993) The neuropathology of temporal lobe epilepsy. J Neuropath Exp Neurol 52: 433-443. - PubMed
-
- Bender RA, Brewster A, Santoro B, Ludwig A, Hofmann F, Biel M, Baram TZ ( 2001) Differential and age-dependent expression of hyperpolarization-activated, cyclic nucleotide-gated cation channel isoforms 1-4 suggests evolving roles in the developing rat hippocampus. Neuroscience 106: 689-698. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
