Brain-derived neurotrophic factor is required for the maintenance of cortical dendrites
- PMID: 12890780
- PMCID: PMC6740724
- DOI: 10.1523/JNEUROSCI.23-17-06856.2003
Brain-derived neurotrophic factor is required for the maintenance of cortical dendrites
Abstract
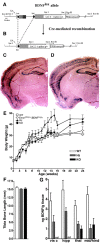
Brain-derived neurotrophic factor (BDNF) is thought to be involved in neuronal survival, migration, morphological and biochemical differentiation, and modulation of synaptic function in the CNS. In the rodent cortex, postnatal BDNF expression is initially low but subsequently increases to reach maximal levels around weaning. Thus, BDNF expression peaks at a time when both structural and functional maturation of cortical circuitry occurs. Although the function of BDNF has been probed using many approaches, its requirements during this phase of life have not previously been examined genetically. To test the in vivo requirements for BDNF during this important phase of development we generated early-onset forebrain-specific BDNF mutant mice. Although these mice undergo forebrain-restricted deletion of BDNF by Cre-mediated recombination during embryogenesis, they are healthy, and we did not detect the loss of specific cortical excitatory or inhibitory neurons. However, the neocortex of 5-week-old mice was thinner, attributable at least partly to neuronal shrinkage. Importantly, although visual cortical layer 2/3 neurons in the mutants initially developed normal dendrite structure, dendritic retraction became apparent by 3 weeks of age. Thus, our observations suggest that cortically expressed BDNF functions to support the maintenance of cortical neuron size and dendrite structure rather than the initial development of these features. This is consistent with a role for BDNF in stabilizing the "survival" of circuitry during the phase of activity-dependent reorganization of cortical connectivity.
Figures




References
-
- Akaneya Y, Tsumoto T, Hatanaka H ( 1996) Brain-derived neurotrophic factor blocks long-term depression in rat visual cortex. J Neurophysiol 76: 4198-4201. - PubMed
-
- Araki T, Yamada M, Ohnishi H, Sano SI, Hatanaka H ( 2000) BIT/SHPS-1 enhances brain-derived neurotrophic factor-promoted neuronal survival in cultured cerebral cortical neurons. J Neurochem 75: 1502-1510. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
