A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer
- PMID: 12890841
- PMCID: PMC2275324
- DOI: 10.1056/NEJMoa021491
A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer
Abstract
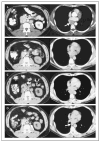
Background: Mutations in the tumor-suppressor gene VHL cause oversecretion of vascular endothelial growth factor by clear-cell renal carcinomas. We conducted a clinical trial to evaluate bevacizumab, a neutralizing antibody against vascular endothelial growth factor, in patients with metastatic renal-cell carcinoma.
Methods: A randomized, double-blind, phase 2 trial was conducted comparing placebo with bevacizumab at doses of 3 and 10 mg per kilogram of body weight, given every two weeks; the time to progression of disease and the response rate were primary end points. Crossover from placebo to antibody treatment was allowed, and survival was a secondary end point.
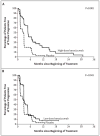
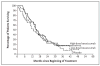
Results: Minimal toxic effects were seen, with hypertension and asymptomatic proteinuria predominating. The trial was stopped after the interim analysis met the criteria for early stopping. With 116 patients randomly assigned to treatment groups (40 to placebo, 37 to low-dose antibody, and 39 to high-dose antibody), there was a significant prolongation of the time to progression of disease in the high-dose--antibody group as compared with the placebo group (hazard ratio, 2.55; P<0.001). There was a small difference, of borderline significance, between the time to progression of disease in the low-dose--antibody group and that in the placebo group (hazard ratio, 1.26; P=0.053). The probability of being progression-free for patients given high-dose antibody, low-dose--antibody, and placebo was 64 percent, 39 percent, and 20 percent, respectively, at four months and 30 percent, 14 percent, and 5 percent at eight months. At the last analysis, there were no significant differences in overall survival between groups (P>0.20 for all comparisons).
Conclusions: Bevacizumab can significantly prolong the time to progression of disease in patients with metastatic renal-cell cancer.
Copyright 2003 Massachusetts Medical Society
Figures
Comment in
-
The von Hippel-Lindau protein, vascular endothelial growth factor, and kidney cancer.N Engl J Med. 2003 Jul 31;349(5):419-21. doi: 10.1056/NEJMp030061. N Engl J Med. 2003. PMID: 12890838 No abstract available.
-
Bevacizumab in renal-cell cancer.N Engl J Med. 2003 Oct 23;349(17):1674. doi: 10.1056/NEJM200310233491719. N Engl J Med. 2003. PMID: 14573745 No abstract available.
-
New antiangiogenic agents for renal cell carcinoma: bevacizumab.Curr Oncol Rep. 2004 Mar;6(2):85-6. doi: 10.1007/s11912-004-0017-2. Curr Oncol Rep. 2004. PMID: 14751083 No abstract available.
References
-
- Gnarra JR, Duan DR, Weng Y, et al. Molecular cloning of the von Hippel-Lindau tumor suppressor gene and its role in renal carcinoma. Biochim Biophys Acta. 1996;1242:201–10. - PubMed
-
- Gnarra JR, Tory K, Weng Y, et al. Mutations of the VHL tumour suppressor gene in renal carcinoma. Nat Genet. 1994;7:85–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
