Ultrastructural distribution of glycinergic and GABAergic neurons and axon terminals in the rat dorsal cochlear nucleus, with emphasis on granule cell areas
- PMID: 12892405
- PMCID: PMC1571146
- DOI: 10.1046/j.1469-7580.2003.00208.x
Ultrastructural distribution of glycinergic and GABAergic neurons and axon terminals in the rat dorsal cochlear nucleus, with emphasis on granule cell areas
Abstract
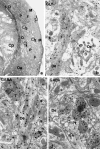
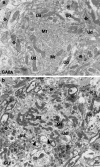
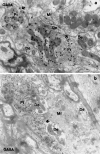
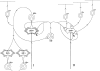
A knowledge of neurotransmitters in the neurons of the rat cochlear nuclear complex is of importance in understanding the function of auditory circuits. Using post-embedding ultrastructural immunogold labelling, the distribution of glycinergic and GABAergic neurons and axonal terminals has been studied in the molecular, fusiform and polymorphic layers of the rat dorsal cochlear nucleus (DCN). This technique is not limited by the penetration of antibodies into the nervous tissue as in pre-embedding methods, and allows a fine neurochemical mapping of the nervous tissue. Numerous glycinergic and GABAergic axon terminals contain pleomorphic and flat synaptic vesicles, and are present in all layers (1, 2, 3) of the dorsal cochlear nucleus. Glycine and GABA-negative large terminals (mossy fibres) are mainly seen in granule cell areas of layer 2 (fusiform layer). Mossy fibres contact the dendrites of GABA- and glycine-negative granule cells and of the few unipolar brush cells (excitatory neurons). The least common cells in the granule cell areas are GABAergic and glycinergic Golgi-stellate neurons. In unipolar brush cells, aggregations of vesicles seem to be the origin of their characteristic ringlet-bodies. Golgi-stellate cells send their inhibitory terminals to the dendrites of granule and unipolar brush cells, occasionally directly to mossy fibres. Small or (less frequently) large GABAergic terminals contact the soma or the main dendrite of unipolar brush cells. The circuit of a hypothetical functional unit of neurons in the DCN is proposed. The inputs from auditory tonotopic or non-auditory non-tonotopic mossy fibres eventually reach pyramidal cells through axons from the granule cells or unipolar brush cells. Pyramidal cells convey an excitatory signal from the DCN to higher mesencephalic nuclei for further elaboration of the acoustic signal.
Figures














Similar articles
-
Mossy fibers in granule cell areas of the rat dorsal cochlear nucleus from intrinsic and extrinsic origin innervate unipolar brush cell glomeruli.J Submicrosc Cytol Pathol. 2004 Apr;36(2):193-210. J Submicrosc Cytol Pathol. 2004. PMID: 15554505
-
Immunocytochemistry of glycine in small neurons of the granule cell areas of the guinea pig dorsal cochlear nucleus: a post-embedding ultrastructural study.Histochem J. 2002 Aug-Sep;34(8-9):423-34. doi: 10.1023/a:1023639621977. Histochem J. 2002. PMID: 12814190
-
Ultrastructural immunocytochemistry for glycine in neurons of the dorsal cochlear nucleus of the guinea pig.J Submicrosc Cytol Pathol. 2003 Oct;35(4):373-87. J Submicrosc Cytol Pathol. 2003. PMID: 15137679
-
Review: cytological characteristics of commissural and tuberculo-ventral neurons in the rat dorsal cochlear nucleus.Hear Res. 2006 Jun-Jul;216-217:73-80. doi: 10.1016/j.heares.2006.01.005. Epub 2006 Feb 28. Hear Res. 2006. PMID: 16510258 Review.
-
The unipolar brush cells of the mammalian cerebellum and cochlear nucleus: cytology and microcircuitry.Prog Brain Res. 1997;114:131-50. doi: 10.1016/s0079-6123(08)63362-2. Prog Brain Res. 1997. PMID: 9193142 Review.
Cited by
-
Effects of cochlear ablation on amino acid levels in the rat cochlear nucleus and superior olive.Hear Res. 2014 Mar;309:44-54. doi: 10.1016/j.heares.2013.11.005. Epub 2013 Nov 28. Hear Res. 2014. PMID: 24291808 Free PMC article.
-
Monaural conductive hearing loss alters the expression of the GluA3 AMPA and glycine receptor α1 subunits in bushy and fusiform cells of the cochlear nucleus.Neuroscience. 2011 Dec 29;199:438-51. doi: 10.1016/j.neuroscience.2011.10.021. Epub 2011 Oct 20. Neuroscience. 2011. PMID: 22044924 Free PMC article.
-
A rapid method combining Golgi and Nissl staining to study neuronal morphology and cytoarchitecture.J Histochem Cytochem. 2008 Jun;56(6):539-50. doi: 10.1369/jhc.2008.950246. Epub 2008 Feb 18. J Histochem Cytochem. 2008. PMID: 18285350 Free PMC article.
-
Single granule cells excite Golgi cells and evoke feedback inhibition in the cochlear nucleus.J Neurosci. 2015 Mar 18;35(11):4741-50. doi: 10.1523/JNEUROSCI.3665-14.2015. J Neurosci. 2015. PMID: 25788690 Free PMC article.
-
Cochlear nucleus neurons redistribute synaptic AMPA and glycine receptors in response to monaural conductive hearing loss.Neuroscience. 2009 Nov 10;163(4):1264-76. doi: 10.1016/j.neuroscience.2009.07.049. Epub 2009 Jul 28. Neuroscience. 2009. PMID: 19646510 Free PMC article.
References
-
- Alibardi L. Characterization of tuberculo-ventral neurons in dorsal cochlear nucleus of the guinea pig. J. Submicrosc. Cytol. Pathol. 1999a;31:295–300. 10.1046/j.1469-7580.2003.00208.x. - DOI - PubMed
-
- Alibardi L. Fine structure, synaptology and immunocytochemistry of large neurons in the rat dorsal cochlear nucleus connected to the inferior colliculus. J. Brain Res. 1999b;39:427–437. 10.1046/j.1469-7580.2003.00208.x. - DOI - PubMed
-
- Alibardi L. Identification of tuberculoventral neurons in the polymorphic layer of the rat dorsal cochlear nucleus. Eur. J. Morphol. 2000a;38:153–166. 10.1046/j.1469-7580.2003.00208.x. - DOI - PubMed
-
- Alibardi L. Cytology of large neurons in the guinea pig dorsal cochlear nucleus. Eur. J. Histochem. 2000b;44:365–375. 10.1046/j.1469-7580.2003.00208.x. - DOI - PubMed
-
- Alibardi L. Cytology, synaptology and immunocytochemistry of commissural neurons and their putative axonal terminals in the dorsal cochlear nucleus of the rat. Ann. Anat. 2000c;182:207–220. 10.1046/j.1469-7580.2003.00208.x. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

