Control of cell number by Drosophila FOXO: downstream and feedback regulation of the insulin receptor pathway
- PMID: 12893776
- PMCID: PMC196255
- DOI: 10.1101/gad.1098703
Control of cell number by Drosophila FOXO: downstream and feedback regulation of the insulin receptor pathway
Abstract
The Drosophila insulin receptor (dInR) regulates cell growth and proliferation through the dPI3K/dAkt pathway, which is conserved in metazoan organisms. Here we report the identification and functional characterization of the Drosophila forkhead-related transcription factor dFOXO, a key component of the insulin signaling cascade. dFOXO is phosphorylated by dAkt upon insulin treatment, leading to cytoplasmic retention and inhibition of its transcriptional activity. Mutant dFOXO lacking dAkt phosphorylation sites no longer responds to insulin inhibition, remains in the nucleus, and is constitutively active. dFOXO activation in S2 cells induces growth arrest and activates two key players of the dInR/dPI3K/dAkt pathway: the translational regulator d4EBP and the dInR itself. Induction of d4EBP likely leads to growth inhibition by dFOXO, whereas activation of dInR provides a novel transcriptionally induced feedback control mechanism. Targeted expression of dFOXO in fly tissues regulates organ size by specifying cell number with no effect on cell size. Our results establish dFOXO as a key transcriptional regulator of the insulin pathway that modulates growth and proliferation.
Figures






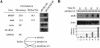
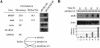










References
-
- Alvarez, B., Martinez, A.C., Burgering, B.M., and Carrera, A.C. 2001. Forkhead transcription factors contribute to execution of the mitotic programme in mammals. Nature 413: 744–747. - PubMed
-
- Ayala, J.E., Streeper, R.S., Desgrosellier, J.S., Durham, S.K., Suwanichkul, A., Svitek, C.A., Goldman, J.K., Barr, F.G., Powell, D.R., and O'Brien, R.M. 1999. Conservation of an insulin response unit between mouse and human glucose-6-phosphatase catalytic subunit gene promoters: Transcription factor FKHR binds the insulin response sequence. Diabetes 48: 1885–1889. - PubMed
-
- Biggin, M.D. and Tjian, R. 1988. Transcription factors that activate the Ultrabithorax promoter in developmentally staged extracts. Cell 53: 699–711. - PubMed
-
- Bohni, R., Riesgo-Escovar, J., Oldham, S., Brogiolo, W., Stocker, H., Andruss, B.F., Beckingham, K., and Hafen, E. 1999. Autonomous control of cell and organ size by CHICO, a Drosophila homolog of vertebrate IRS1-4. Cell 97: 865–875. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
