The sex peptide of Drosophila melanogaster: female post-mating responses analyzed by using RNA interference
- PMID: 12893873
- PMCID: PMC187888
- DOI: 10.1073/pnas.1631635100
The sex peptide of Drosophila melanogaster: female post-mating responses analyzed by using RNA interference
Abstract
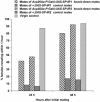
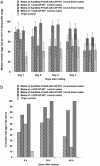
Mating induces profound changes in female insect behavior and physiology. In Drosophila melanogaster, mating causes a reduction in sexual receptivity and an elevation in egg production for at least 5 days. Injection of the seminal fluid sex peptide (SP) induces both responses in virgin females, but only for 1-2 days. The role of SP in eliciting the responses to mating remains to be elucidated. Functional redundancy between seminal fluid components may occur. In addition, mating with spermless males results in brief (1- to 2-day) post-mating responses, indicating either that there is a "sperm effect" or that sperm act as carriers for SP or other seminal fluid components. Here we used RNA interference to suppress SP expression, to determine whether SP is required to elicit full post-mating responses, the magnitude of responses due to other seminal fluid components, and whether SP accounts for the "sperm effect." Receptivity was higher and egg production lower in females mated to SP knock-down males than in controls. Comparison with virgins showed that the responses were brief. SP is therefore required for normal magnitude and persistence of postmating responses. Sperm transfer and use were normal in mates of SP knock-down males, yet their post-mating responses were briefer than after normal matings, and similar to those reported in mates of spermless son-of-tudor males. The prolonged "sperm effect" on female receptivity and egg production is therefore entirely attributable to SP, but sperm are necessary for its occurrence.
Figures
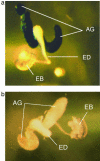


Comment in
-
Sex peptide and the sperm effect in Drosophila melanogaster.Proc Natl Acad Sci U S A. 2003 Aug 19;100(17):9643-4. doi: 10.1073/pnas.1834127100. Epub 2003 Aug 11. Proc Natl Acad Sci U S A. 2003. PMID: 12913117 Free PMC article. No abstract available.
References
-
- Chen, P. S. (1984) Annu. Rev. Entomol. 29, 233–255.
-
- Gillot, C. (2003) Annu. Rev. Entomol. 48, 163–184. - PubMed
-
- Miller, J. R., Spencer, J. L., Lentz, A. J., Keller, J. E., Walker, E. D. & Leykam, J. F. (1994) ACS Symp. Ser. 551, 189–209.
-
- Partridge, L. (1996) in Fruit Fly Pests: A World Assessment of Their Biology and Management, eds. McPheron, B. A. & Steck, G. J. (St. Lucie Press, Boca Raton, FL), pp. 9–15.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

